| 参考文献No. | 482608 |
| 标题: | Synthesis and methemoglobin toxicity of the amides of 6/7 mono or disubstituted quinolone |
| 作者: | Srivastava, S.; Srivastava, S.K.; Shukla, A.; Chauhan, P.M.; Puri, S.K.; Bhaduri, A.P.; Pandey, V.C. |
| 来源: | Bioorg Med Chem Lett 1999,9(1),25 |
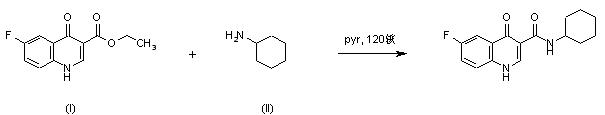 |
| 合成路线图解说明: Title compound was synthesized by condensation of ethyl 6-fluoro-4-quinolone-3-carboxylate (I) with cyclohexylamine (II) in pyridine at 120 C under pressure. |
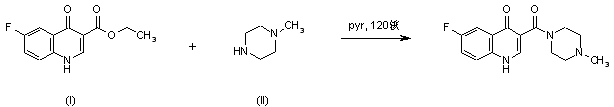 |
| 合成路线图解说明: Title compound was synthesized by condensation of ethyl 6-fluoro-4-quinolone-3-carboxylate (I) with N-methylpiperazine (II) in pyridine at 120 C under pressure. |
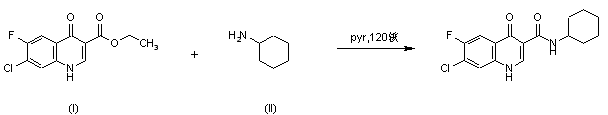 |
| 合成路线图解说明: Title compound was synthesized by condensation of ethyl 7-chloro-6-fluoro-4-quinolone-3-carboxylate (I) with cyclohexylamine (II) in pyridine at 120 C under pressure. |
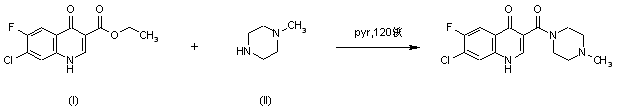 |
| 合成路线图解说明: Title compound was synthesized by condensation of ethyl 7-chloro-6-fluoro-4-quinolone-3-carboxylate (I) with N-methylpiperazine (II) in pyridine at 120 C under pressure. |