| 参考文献No. | 25978 |
| 标题: | Indoline derivs. as 5HT2C antagonists |
| 作者: | Ham, P.; Jones, G.E.; Forbes, I.T. (SmithKline Beecham plc) |
| 来源: | EP 0707581; JP 1996512299; US 5834494; WO 9501976 |
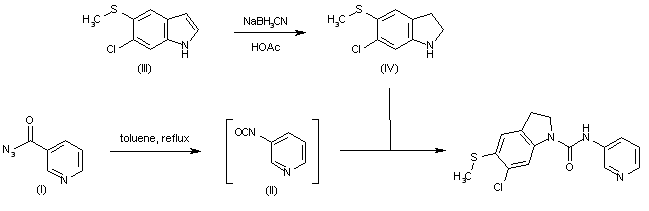 |
| 合成路线图解说明: The heating of nicotinoyl azide (I) in refluxing toluene gives the corresponding isocyanate (II), which without isolation is condensed with 6-chloro-5-(methylsulfanyl)indoline (IV) to afford the target compound. Intermediate indoline (IV) is obtained by reduction of 6-chloro-5-(methylsulfanyl)indole (III) with NaBH3CN in HOAc. |
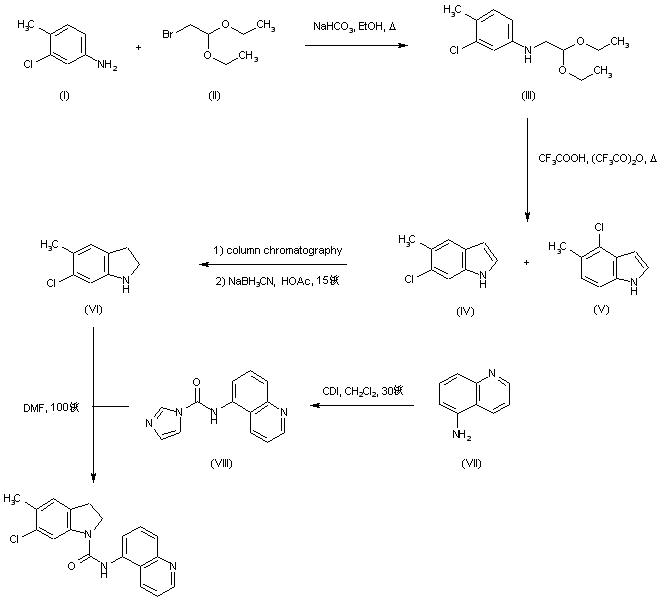 |
| 合成路线图解说明: Alkylation of 3-chloro-4-methylaniline (I) with bromoacetaldehyde diethyl acetal (II) afforded secondary amine (III). Subsequent cyclization of (III) in boiling trifluoroacetic acid/trifluoroacetic anhydride provided a (3:2) mixture of indoles (IV) and (V), from which the desired 6-chloro isomer (IV) was isolated by column chromatography, and then reduced to indoline (VI) using NaBH3CN in AcOH. 5-Aminoquinoline (VII) was treated with carbonyl diimidazole to give intermediate (VIII). This was finally coupled with indoline (VI) in DMF at 100 C to yield the corresponding urea. |