| 参考文献No. | 41006 |
| 标题: | N6-Substd. nucleotide analogues and their use |
| 作者: | Holy, A.; De Clercq, E.D.A. (Institute of Organic Chemistry and Biochemistry; Stichting Rega Vzw) |
| 来源: | US 5977061; WO 9633200 |
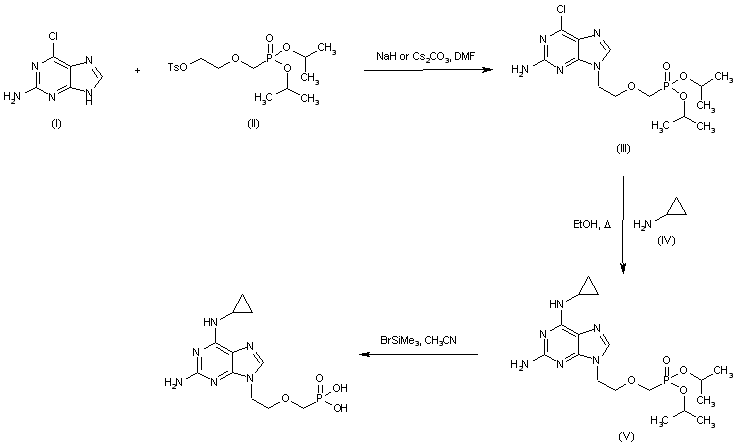 |
| 合成路线图解说明: Alkylation of 2-amino-6-chloropurine (I) with tosylate (II) afforded the phosphonomethoxyethyl purine (III). Subsequent displacement of the chloro atom of (III) by cyclopropylamine (IV) furnished diaminopurine (V). The isopropyl protecting groups of (IV) were finally cleaved by treatment with bromotrimethylsilane. |
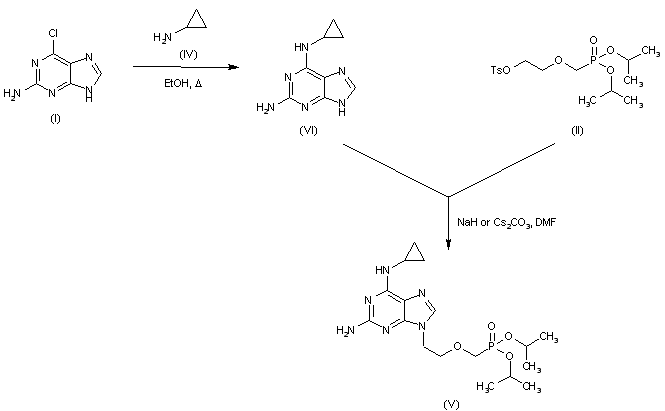 |
| 合成路线图解说明: In an alternative method for preparing precursor (V), 2-amino-6-chloropurine (I) was first treated with cyclopropylamine (IV) to give diaminopurine (VI), and then alkylated with tosylate (II). |