| 参考文献No. | 543242 |
| 标题: | Total synthesis of HUN-7293 |
| 作者: | Boger, D.L.; et al. |
| 来源: | J Am Chem Soc 1999,121(26),6197 |
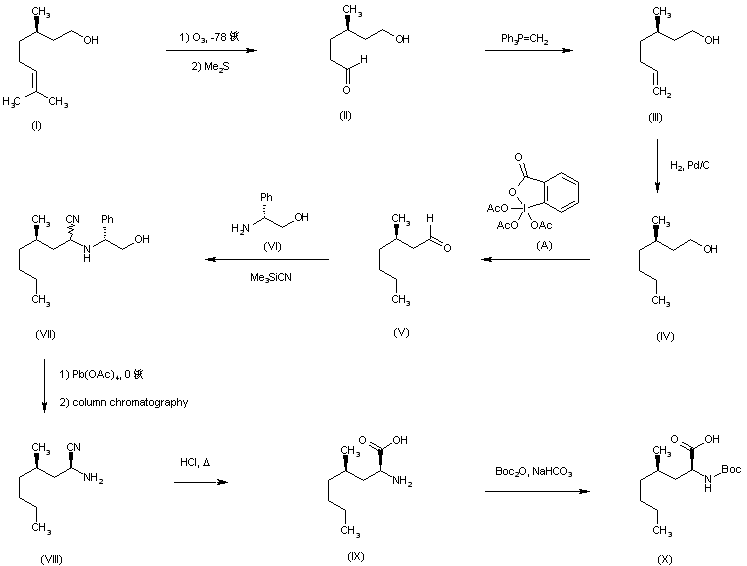 |
| 合成路线图解说明: The preparation of the precursor amino acids is shown in Schemes 1-4. Ozonolysis of (R)-citronellol (I), followed by Wittig reaction of the resulting aldehyde (II) with methylene triphenylphosphorane gave heptenol (III). Subsequent hydrogenation of (III) over Pd/C provided the saturated alcohol (IV), which was oxidized to the corresponding aldehyde (V) by treatment with Dess-Martin reagent. Asymmetric Strecker reaction in (V) utilizing (R)-phenylglycinol (VI) and trimethylsilyl cyanide afforded a diastereomeric mixture of aminonitriles (VII). After oxidative cleavage of (VII) with lead tetraacetate, the free (2S)-amine (VIII) was separated from its minor diastereomer by column chromatography. Acid hydrolysis of the nitrile group of (VIII) gave 5-propyl-L-leucine (IX), which was protected as the tert-butyl carbamate (X) with Boc2O. |
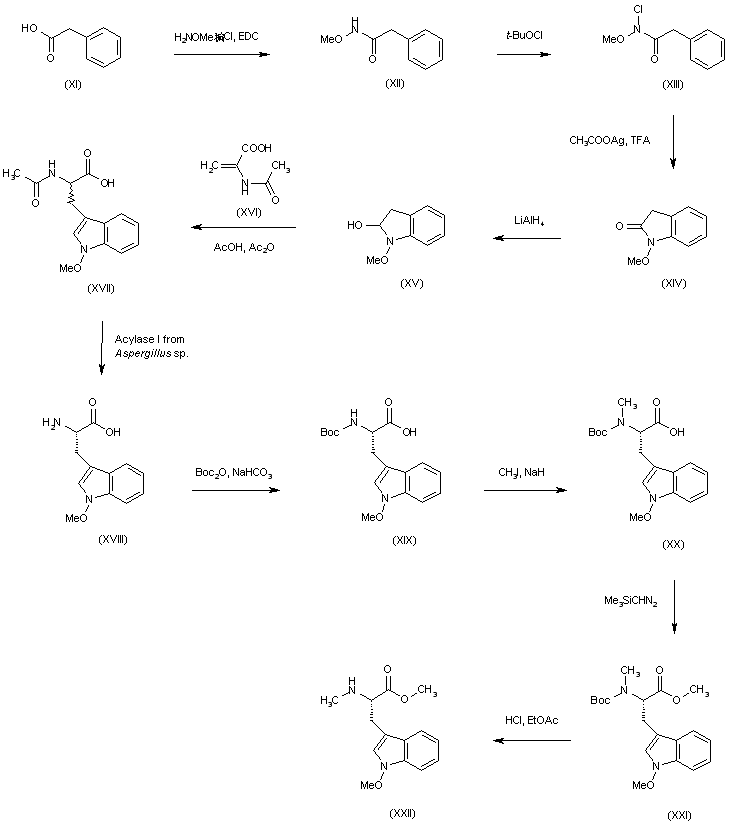 |
| 合成路线图解说明: Condensation of phenylacetic acid (XI) with methoxyamine gave hydroxamate (XII). After N-chlorination of (XII) with tert-butyl hypochlorite, Ag(I)-catalyzed ring closure of the intermediate (XIII) produced N-(methoxy)oxindole (XIV). Amide reduction of (XIV) with LiAlH4 gave hemiaminal (XV), which was converted to the racemic tryptophan derivative (XVII) by reaction with alpha-acetamidoacrylic acid (XVI) in AcOH-Ac2O. Optical resolution of (XVII) by enantioselective enzymatic hydrolysis of the acetamide group using Acylase I from Aspergillus sp. afforded N1'-methoxy-L-tryptophan (XVIII), which was protected as the N-Boc derivative (XIX) and N-methylated by means of MeI and NaH yielding (XX). The resulting N-methyl amino acid (XX) was esterified upon treatment with (trimethylsilyl)diazomethane giving (XXI), and the N-Boc group was removed with HCl in EtOAc to provide the tryptophan derivative (XXII). |
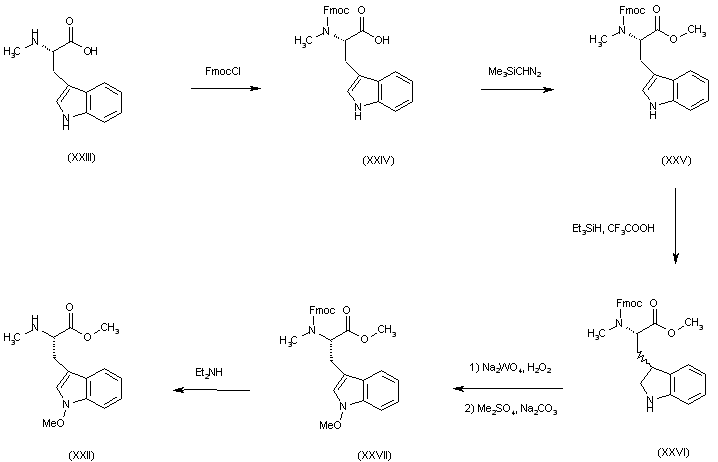 |
| 合成路线图解说明: A more effective preparation of (XXII) was further developed starting from N-methyltryptophan (XXIII). Protection as the N-Fmoc derivative (XXIV) and then esterification with (trimethylsilyl)diazomethane yielded (XXV). Reduction of (XXV) with triethylsilane and TFA produced indoline (XXVI), which was reoxidized to the corresponding N-hydroxyindole with Na2WO4 and H2O2, and then O-methylated with Me2SO4 to give (XXVII). Finally, Fmoc deprotection of (XXVII) with Et2NH provided aminoester (XXII). |
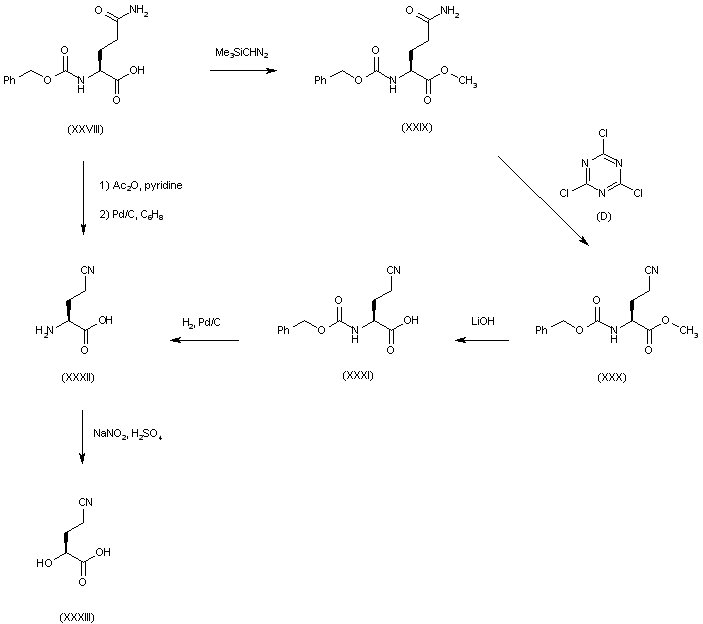 |
| 合成路线图解说明: Treatment of N-(benzyloxycarbonyl)-L-glutamine (XXVIII) with (trimethylsilyl)-diazomethane provided methyl ester (XXIX). Nitrile (XXX) was then obtained from (XXIX) by dehydration with cyanuryl chloride. Hydrolysis of the methyl ester of (XXX) with LiOH gave (XXXI), and hydrogenolysis of the carbobenzoxy group provided amino acid (XXXII). Alternatively, amino acid (XXXII) could be obtained by direct amide dehydration of (XXVIII) with Ac2O and pyridine, followed by transfer hydrogenolysis of the Cbz protecting group. Diazotization of amino acid (XXXII) with NaNO2 in the presence of H2SO4 then furnished (S)-2-hydroxy-4-cyanobutyric acid (XXXIII). |
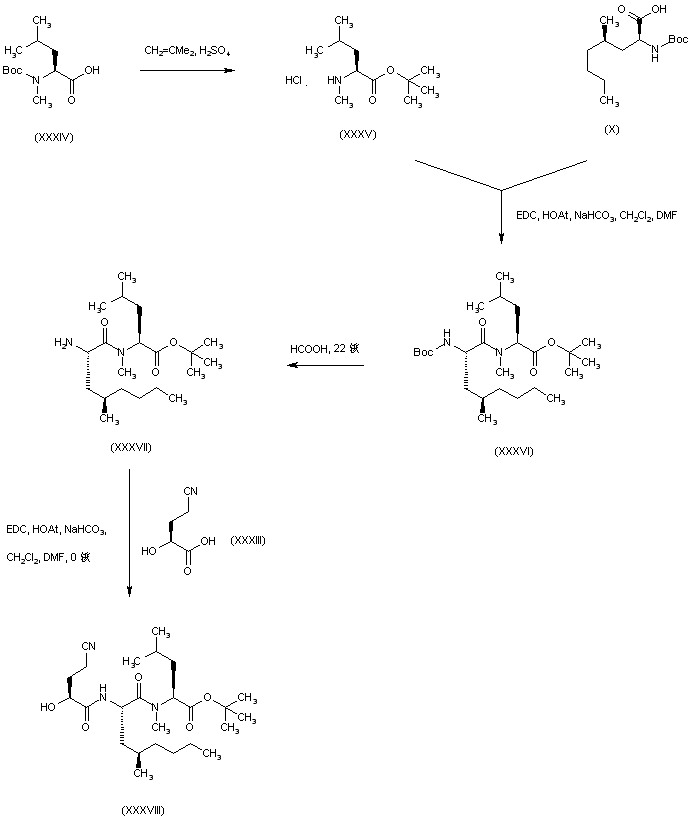 |
| 合成路线图解说明: Treatment of N-Boc-N-methyl-L-leucine (XXXIV) with isobutene and H2SO4 in CH2Cl2 generated tert-butyl ester (XXXV) with simultaneous removal of the Boc group. Subsequent coupling of (XXXV) with N-Boc-5-propyl-L-leucine (X) utilizing EDC and HOAt provided dipeptide (XXXVI). Selective removal of the N-Boc group of (XXXVI) was accomplished by treatment with formic acid at r.t. The resulting amine (XXXVII) was then coupled with hydroxyacid (XXXIII) to yield the target tripeptide (XXXVIII). |
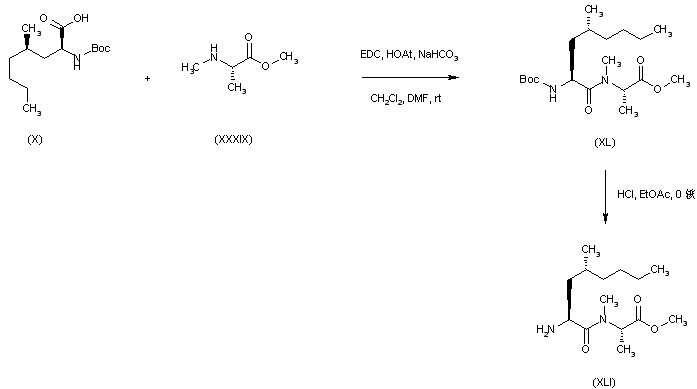 |
| 合成路线图解说明: Coupling of N-Boc-5-propyl-L-leucine (X) with N-methyl-L-alanine methyl ester (XXXIX) in the presence of EDC and HOAt gave dipeptide (XL), which was deprotected with HCl in EtOAc to afford (XLI). |
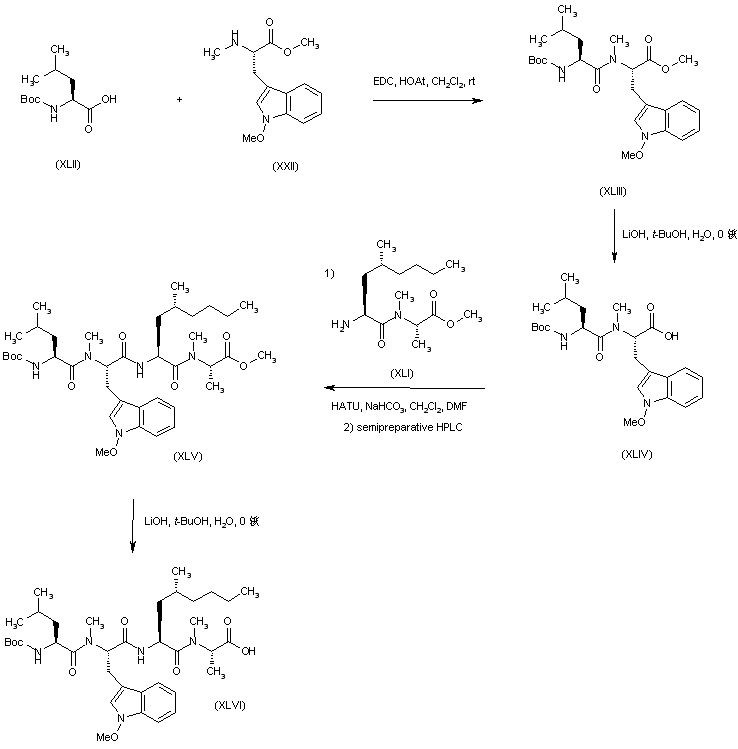 |
| 合成路线图解说明: Dipeptide (XLIV) was prepared by coupling N-Boc-L-leucine (XLII) with tryptophan derivative (XXII), followed by basic hydrolysis of the resulting dipeptide ester (XLIII). Dipeptides (XLIV) and (XLI) were then coupled in the presence of HATU to afford tetrapeptide (XLV), which was separated from minor amounts of a tryptophan-epimerized byproduct by semipreparative RP-HPLC. Tetrapeptide ester (XLV) was then hydrolyzed to acid (XLVI) with LiOH in aqueous tert-butanol. |
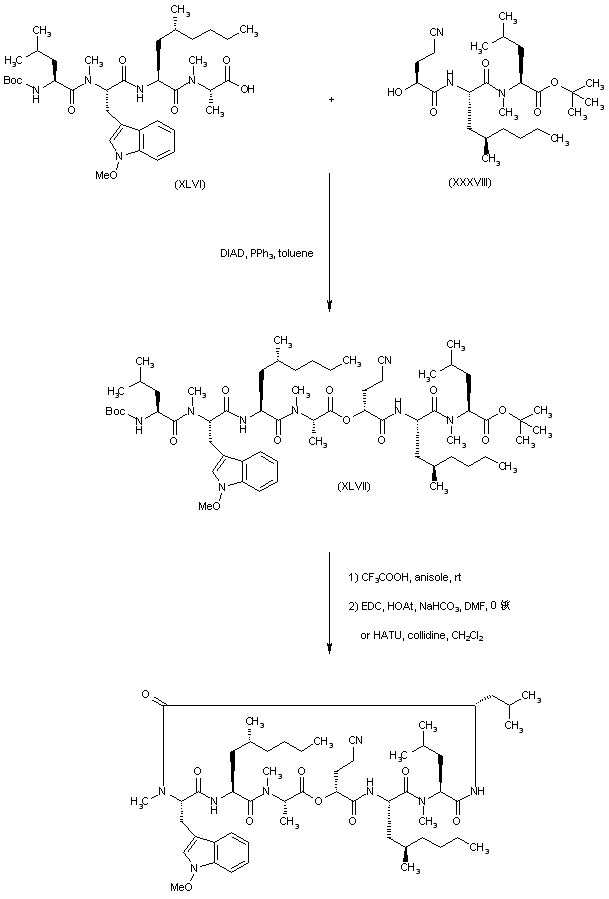 |
| 合成路线图解说明: The condensation of tetrapeptide (XLVI) with tripeptide alcohol (XXXVIII) under modified Mitsunobu conditions furnished the linear heptadepsipeptide (XLVII). Removal of both Boc group and tert-butyl ester was accomplished by treatment with TFA at r.t. Finally, cyclization to the target compound was performed either with EDC and HOAt or with HATU and collidine. |