| 参考文献No. | 569610 |
| 标题: | Total synthesis of macrosphelides B and A |
| 作者: | Kobayashi, Y.; et al. |
| 来源: | Tetrahedron Lett 2000,41(10),1559 |
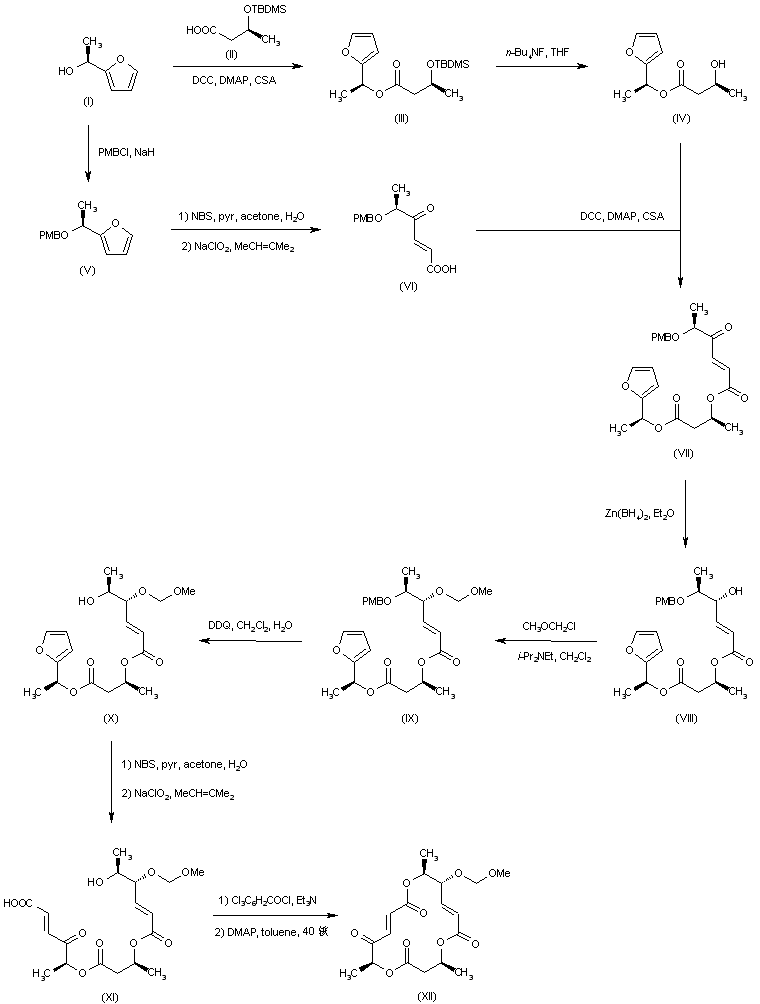 |
| 合成路线图解说明: Coupling of (S)-1-(2-furyl)ethanol (I) with the silylated (S)-3-hydroxybutyric acid (II) afforded ester (III). The silyl protecting group of (III) was then removed by treatment with tetrabutylammonium fluoride to provide the intermediate hydroxy ester (IV). Keto acid (VI) was prepared from the protected furylethanol (V) by oxidative cleavage of the furan ring with N-bromosuccinimide, and further oxidation of the intermediate aldehyde with sodium chlorite. Coupling of keto acid (VI) with hydroxy ester (IV) furnished the corresponding ester (VII). Ketone reduction in (VII) with Zn(BH4)2 yielded alcohol (VIII), which was subsequently protected as the methoxymethyl ether (IX). The PMB group of (IX) was then deprotected by treatment with DDQ to give alcohol (X). Oxidative cleavage of the furan ring of (X) as above gave rise to keto acid (XI). Cyclization to produce the macrolactone (XII) was achieved via activation with trichlorobenzoyl chloride. |
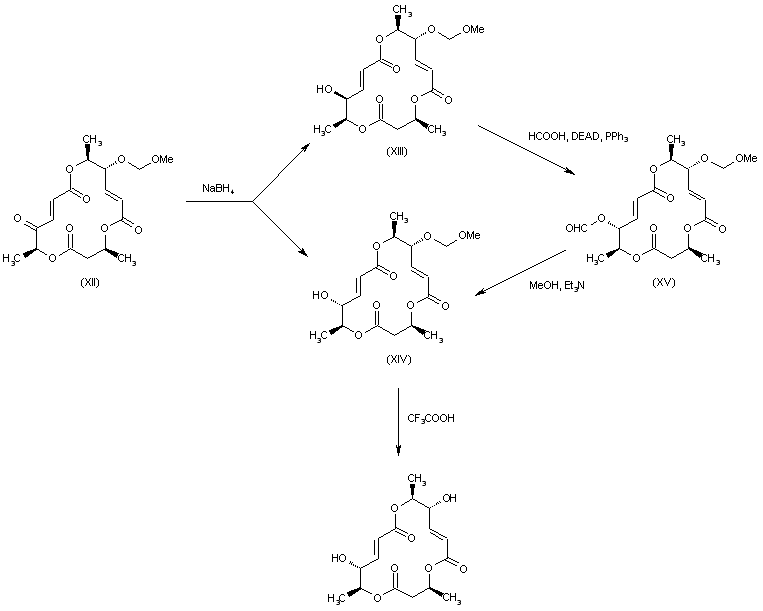 |
| 合成路线图解说明: Borohydride reduction of the 14-keto group of (XII) produced a mixture of the desired alcohol (XIV) and its epimer (XV). Inversion of the configuration of the undesired alcohol (XV) was carried out by Mitsunobu coupling with formic acid, followed by methanolysis of the resulting formate ester (XV) with MeOH and Et3N. The MOM protecting group of (XIV) was finally cleaved by treatment with trifluoroacetic acid. |
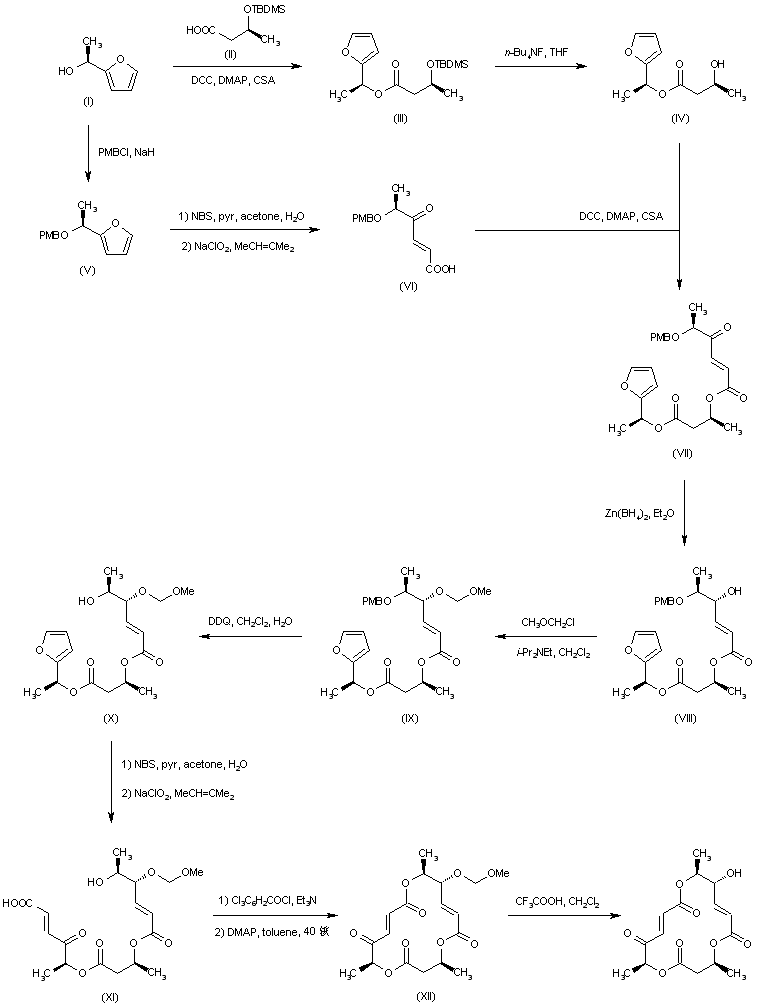 |
| 合成路线图解说明: Coupling of (S)-1-(2-furyl)ethanol (I) with the silylated (S)-3-hydroxybutyric acid (II) afforded ester (III). The silyl protecting group of (III) was then removed by treatment with tretrabutylammonium fluoride to provide the intermediate hydroxy ester (IV). Keto acid (VI) was prepared from the protected furylethanol (V) by oxidative cleavage of the furan ring with N-bromosuccinimide, and further oxidation of the intermediate aldehyde with sodium chlorite. Coupling of keto acid (VI) with hydroxy ester (IV) furnished the corresponding ester (VII). Ketone reduction in (VII) with Zn(BH4)2 yielded alcohol (VIII), which was subsequently protected as the methoxymethyl ether (IX). The PMB group of (IX) was then deprotected by treatment with DDQ to give alcohol (X). Oxidative cleavage of the furan ring of (X) as above gave rise to keto acid (XI). Cyclization of (XI) to produce the macrolactone (XII) was achieved via activation with trichlorobenzoyl chloride. The MOM protecting group of (XII) was finally cleaved by treatment with trifluoroacetic acid. |