| 参考文献No. | 17722 |
| 标题: | Heteroaroyl derivs. of monocyclic beta-lactam antibiotics |
| 作者: | Koster, W.H.; Sundeen, J.E.; Straub, H.; Ermann, P.H.; Treuner, U. (Bristol-Myers Squibb Co.) |
| 来源: | EP 0484881; JP 1992283579 |
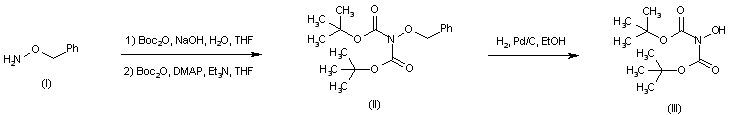 |
| 合成路线图解说明: Sequential introduction of two tert-butoxycarbonyl groups into O-benzyl hydroxylamine (I) afforded the di-Boc-protected hydroxylamine (II). The O-benzyl group was then removed by hydrogenolysis over Pd/C yielding intermediate (III). |
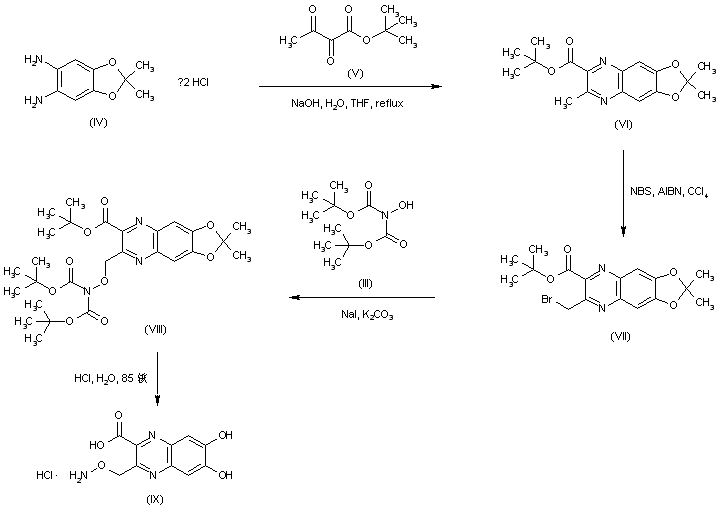 |
| 合成路线图解说明: Quinoxaline (VI) was prepared by condensation of phenylenediamine (IV) with tert-butyl 2,3-dioxobutyrate (V). Radical bromination of (VI) using N-bromosuccinimide and AIBN furnished the bromomethyl quinoxaline (VII) as the major brominated compound. This was condensed with N,N-di-Boc-hydroxylamine (III) to afford the protected O-alkyl hydroxylamine (VIII). Then, treatment of (VIII) with hot concentrated HCl gave rise to the fully deprotected quinoxaline (IX). |
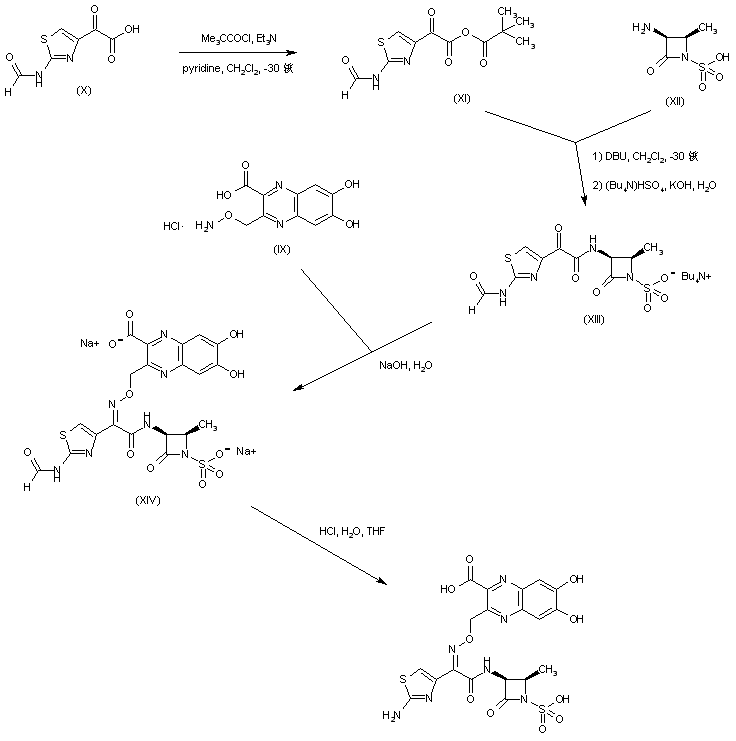 |
| 合成路线图解说明: 2-Formamido-4-thiazolecarboxylic acid (X) was activated as the mixed anhydride (XI) upon treatment with pivaloyl chloride and triethylamine. Subsequent coupling of anhydride (XI) with the aminoazetidinesulfonic acid (XII) provided the corresponding amide, which was further converted to the tetrabutylammonium sulfamate salt (XIII). The condensation between the keto group of (XIII) and hydroxylamine (IX) gave rise to the corresponding oxime (XIV). Finally, acid hydrolysis of the N-formyl group of (XIV) furnished the title compound. |
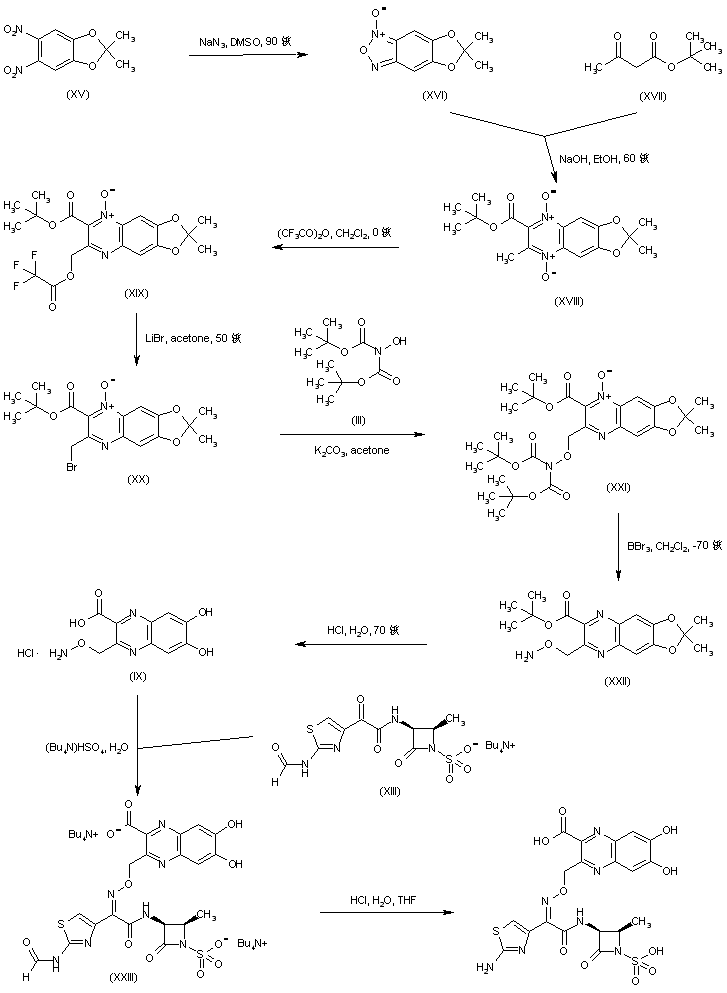 |
| 合成路线图解说明: In an alternative route to the title compound, dinitro derivative (XV) was converted into the benzofuroxan (XVI) by treatment with sodium azide in hot DMSO. Furazan ring opening by means of tert-butyl acetoacetate (XVII) under basic conditions generated the quinoxaline N,N'-dioxide (XVIII). The Pummerer-like rearrangement of the N-oxide (XVIII) in the presence of trifluoroacetic anhydride gave rise to the unstable trifluoroacetoxymethyl quinoxaline (XIX), which was further converted to bromide (XX) upon heating with LiBr in acetone. Alkylation of N,N-di-Boc-hydroxylamine (III) with bromide (XX) provided the protected O-alkyl hydroxylamine (XXI). Reduction of the remaining N-oxide group of (XXI) and simultaneous N-Boc groups cleavage by means of boron tribromide furnished quinoxaline (XXII). The fully deprotected quinoxaline (IX) was then obtained by acidic treatment of (XXII). Condensation of keto amide (XIII) with hydroxylamine (IX) in the presence of tetrabutylammonium bisulfate produced the oxime adduct (XXIII). Finally, the title compound was obtained by deformylation of (XXIII) under acidic conditions. |