| 参考文献No. | 562897 |
| 标题: | Synthetic routes to quinoline derivatives: Novel syntheses of 3-butyryl-8-methoxy-4-[(2-methylphenyl)amini]quinoline and 3-butyryl-8-(2-hydroxyethoxy)-4-[(2-methylphenyl)amino]quin |
| 作者: | Atkins, R.J.; et al. |
| 来源: | Org Process Res Dev 1997,1(3),185 |
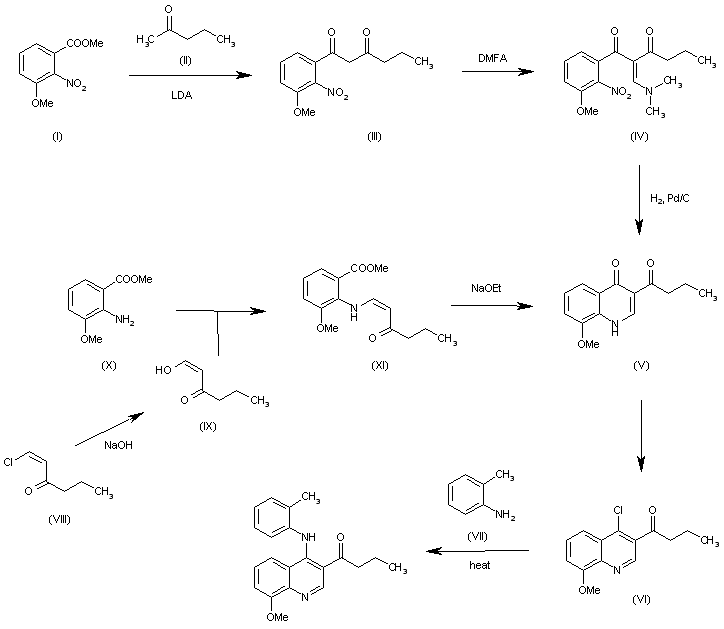 |
| 合成路线图解说明: The reaction of 3-methoxy-2-nitrobenzoic acid methyl ester (I) with 2-pentanone (II) by means of LDA gives 1-(3-methoxy-2-nitrophenyl)hexane-1,3-dione (III), which is condensed with dimethylformamide dimethyl acetal (DMFA) yielding the enamine (IV). The cyclization of (IV) by hydrogenation with H2 over Pd/C affords quinolone (V), which is treated with refluxing POCl3 to give 1-(4-chloro-8-methoxyquinolin-3-yl)-1-butanone (VI). Finally, this compound is condensed with o-toluidine in refluxing dioxane.
The intermediate, the quinolone (V) has also been obtained as follows: The reaction of 1-chloro-1-hexen-3-one (VIII) with NaOH in methanol gives 3-oxohexanal (IX) (enol form), which is condensed with 2-amino-3-methoxybenzoic acid methyl ester (X) yielding the enamine (XI). Finally, this compound is cyclized by means of sodium methoxide to afford the desired quinolone (V). |
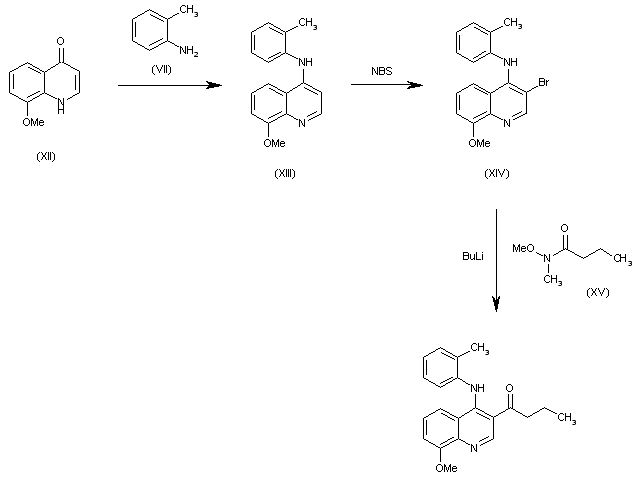 |
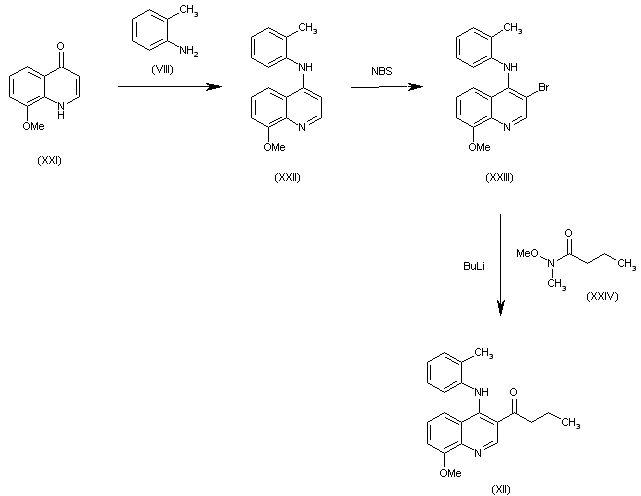
| 合成路线图解说明: The condensation of 8-methoxyquinolin-4(1H)-one (XII) with o-toluidine (VII) gives the secondary amine (XIII), which is brominated with NBS to the 3-bromo derivative (XIV). Finally, this compound is condensed with N-methoxy-N-methylbutyramide (XV) by means of BuLi to afford the target compound. |
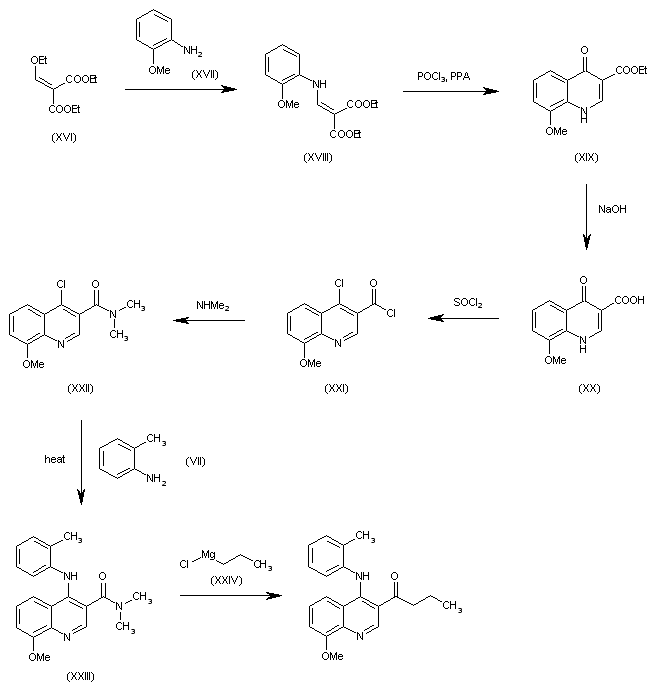 |
| 合成路线图解说明: The condensation of ethoxymethylenemalonic acid diethyl ester (XVI) with 2-methoxyaniline (XVII) in hot toluene gives the aminomethylene derivative (XVIII), which is cyclized with POCl3 and polyphosphoric acid (PPA) at 100 C yielding 8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid ethyl ester (XIX), which is hydrolyzed with NaOH in methanol to the corresponding free acid (XX), and treated with refluxing SOCl2 to afford 4-chloro-8-methoxyquinoline-3-carbonyl chloride (XXI). The reaction of (XXI) with dimethylamine gives the corresponding amide (XXII), which is condensed with o-toluidine (VII) in refluxing dioxane yielding the expected secondary amine (XXIII). Finally, this compound is treated with propylmagnesium chloride (XXIV) to afford the target compound. |
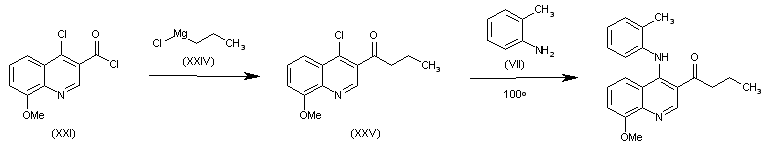 |
| 合成路线图解说明: The reaction of acyl chloride (XXI) with propylmagnesium chloride (XXIV) gives 1-(4-chloro-8-methoxyquinolin-3-yl)-1-butanone (XXV), which is finally condensed with o-toluidine (VII) in refluxing dioxane to afford the target compound. |
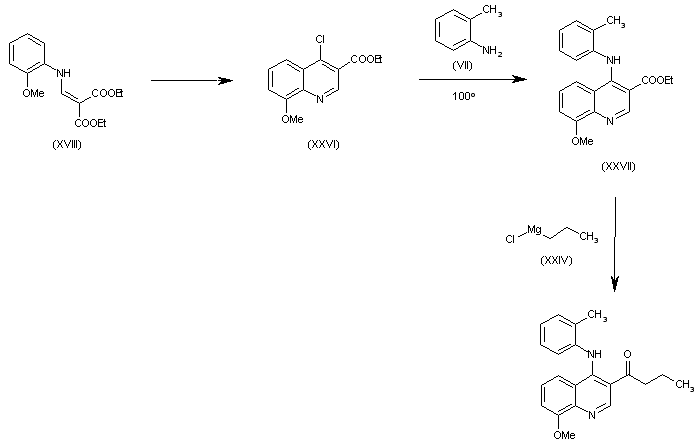 |
| 合成路线图解说明: The cyclization of (XVIII) in refluxing POCl3 gives 4-chloro-8-methoxyquinoline-3-carboxylic acid ethyl ester (XXVI), which is condensed with o-toluidine (VII) in refluxing dioxane yielding the secondary amine (XXVII). Finally, this compound is condensed with propylmagnesium chloride (XXIV) to afford the target compound. |
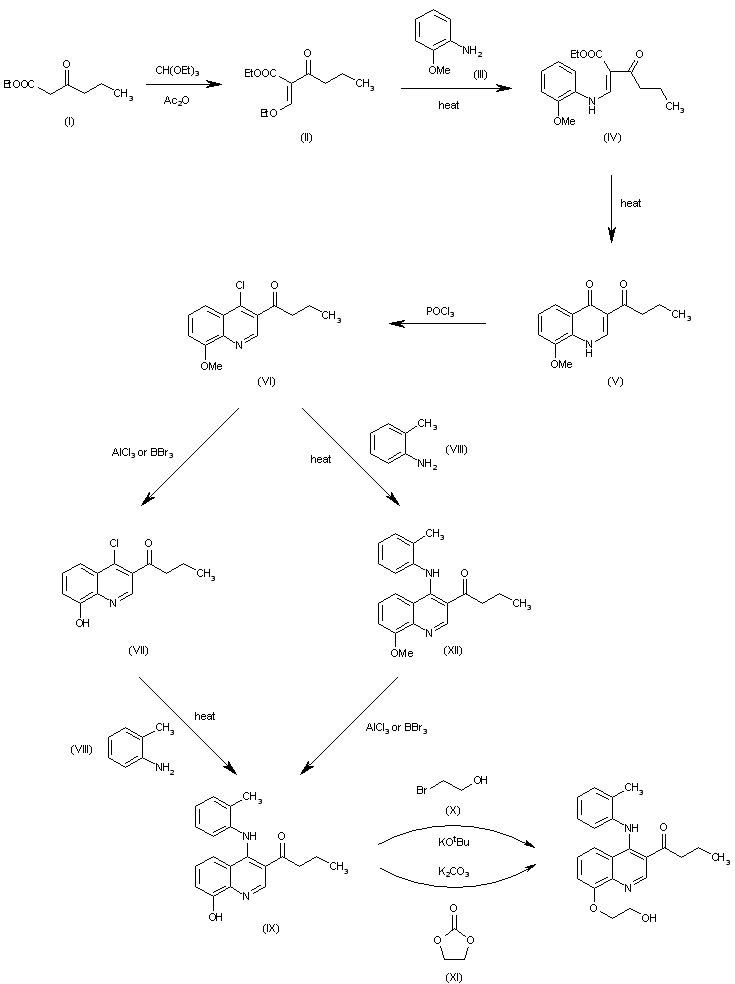 |
| 合成路线图解说明: This compound has been obtained by several related ways:
1) The reaction of ethyl butyrylacetate (I) with triethyl orthoformate and Ac2O gives acrylate (II), which by reaction with o-toluidine (III) yields the aminoacrylate (IV). The cyclization of (IV) by heating at 255 C in diphenyl ether affords the quinolone (V), which is treated with refluxing POCl3 to give 1-(4-chloro-8-methoxyquinolin-3-yl)-1-butanone (VI). The demethylation of (VI) with AlCl3 or BBr3 yields the 8-hydroxyquinoline (VII), which is condensed with o-toluidine (VIII) in refluxing dioxane affording 1-[8-hydroxy-4-(2-methylphenylamino)quinolin-3-yl-1-butanone (IX). Finally, this compound is condensed with 2-bromomethanol (X) by means of potassium tert-butoxide or with ethylene carbonate (XI) by means of K2CO3.
2) The condensation of chloroquinoline (VI) with o-toluidine (VIII) in refluxing dioxane gives 1-[8-methoxy-4-(2-methylphenylamino)quinolin-3-yl-1-butanone (XII), which is demethylated with AlCl3 or BBr3 as before yielding the previously reported 1-[8-hydroxy-4-(2-methylphenylamino)quinolin-3-yl-1-butanone (IX). |
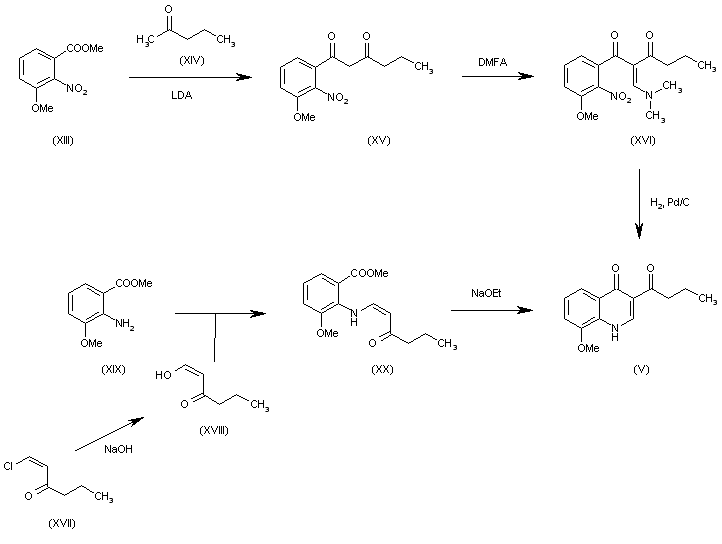 |
| 合成路线图解说明: The intermediate quinolone (V) has also been obtained by two other ways:
a) The reaction of 3-methoxy-2-nitrobenzoic acid methyl ester (XIII) with 2-pentanone (XIV) by means of LDA gives 1-(3-methoxy-2-nitrophenyl)hexane-1,3-dione (XV), which is condensed with dimethylformamide dimethyl acetal (DMFA) yielding the enamine (XVI). Finally, this compound is cyclized by hydrogenation with H2 over Pd/C to afford the desired quinolone (V).
b) The reaction of 1-chloro-1-hexen-3-one (XVII) with NaOH in methanol gives 3-oxohexanal (XVIII) (enol form), which is condensed with 2-amino-3-methoxybenzoic acid methyl ester (XIX) yielding the enamine (XX). Finally, this compound is cyclized by means of sodium methoxide to afford the desired quinolone (V). |
 |
| 合成路线图解说明: The condensation of 8-methoxyquinolin-4(1H)-one (XXI) with o-toluidine (VIII) gives the secondary amine (XXII), which is brominated with NBS to the 3-bromo derivative (XXIII). Finally, this compound is condensed with N-methoxy-N-methylbutyramide (XXIV) by means of BuLi to afford intermediate (XII). |
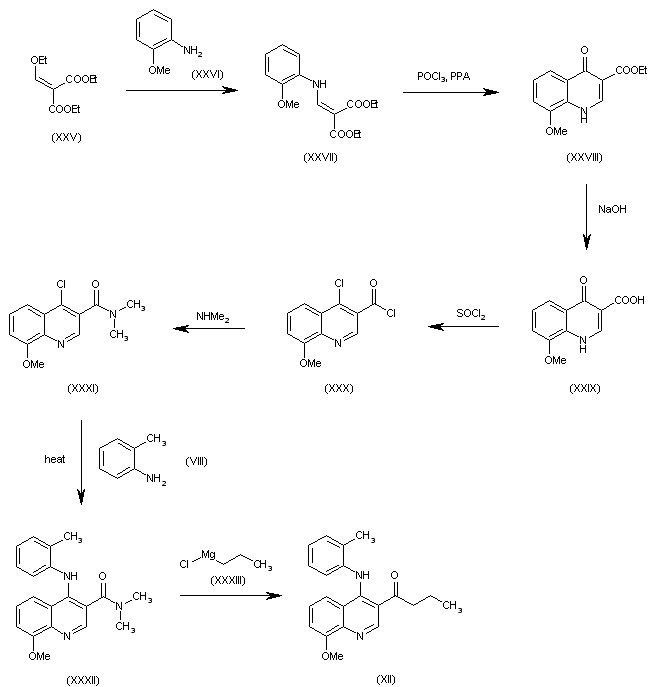 |
| 合成路线图解说明: The condensation of ethoxymethylenemalonic acid diethyl ester (XXV) with 2-methoxyaniline (XXVI) in hot toluene gives the aminomethylene derivative (XXVII), which is cyclized with POCl3 and polyphosphoric acid (PPA) at 100 C yielding 8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid ethyl ester (XXVIII), which is hydrolyzed with NaOH in methanol to the corresponding free acid (XXIX), and treated with refluxing SOCl2 to afford 4-chloro-8-methoxyquinoline-3-carbonyl chloride (XXX). The reaction of (XXX) with dimethylamine gives the corresponding amide (XXXI), which is condensed with o-toluidine (VIII) in refluxing dioxane yielding the expected secondary amine (XXXII). Finally, this compound is treated with propylmagnesium chloride (XXXIII) to afford the intermediate (XII). |
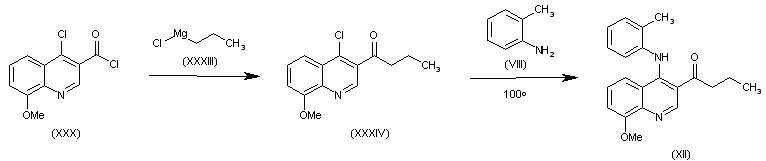 |
| 合成路线图解说明: The reaction of acyl chloride (XXX) with propylmagnesium chloride (XXXIII) gives 1-(4-chloro-8-methoxyquinolin-3-yl)-1-butanone (XXXIV), which is finally condensed with o-toluidine (VIII) in refluxing dioxane to afford the intermediate (XII). |
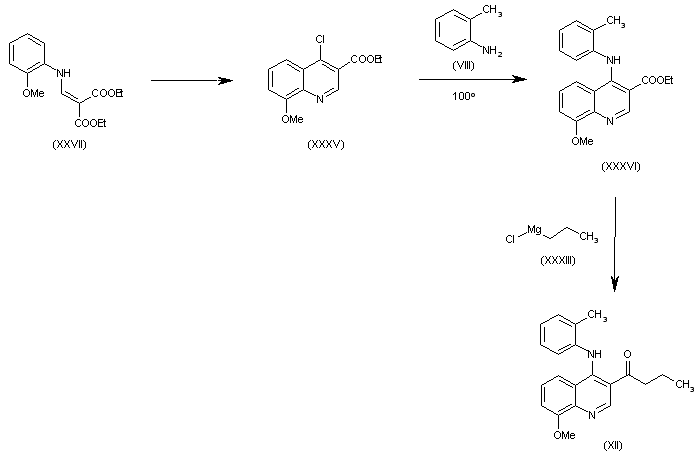 |
| 合成路线图解说明: The cyclization of (XXVII) in refluxing POCl3 gives 4-chloro-8-methoxyquinoline-3-carboxylic acid ethyl ester (XXXV), which is condensed with o-toluidine (VIII) in refluxing dioxane yielding the secondary amine (XXXVI). Finally, this compound is condensed with propylmagnesium chloride (XXXIII) to afford the intermediate (XII). |