| 参考文献No. | 9150 |
| 标题: | Benzhydryloxyethylpiperidine derivs., process for their preparation and pharmaceutical compsns., in which they are present |
| 作者: | Buzas, A.; Merour, J.-Y.; Ollivier, R. (Laboratoires Meram SA) |
| 来源: | EP 0259227; US 4983614 |
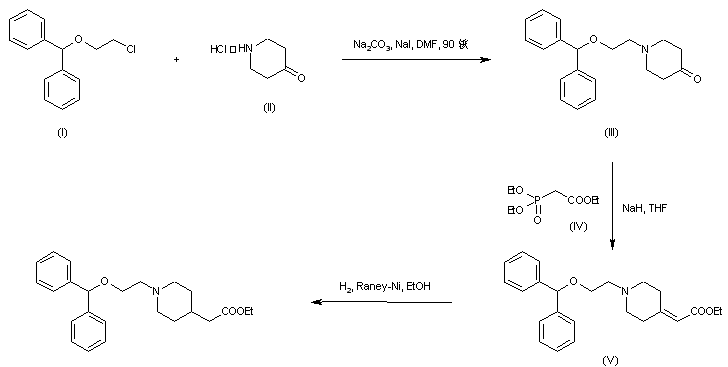 |
| 合成路线图解说明: The title compound was obtained by two related procedures. Reaction of 4-piperidone-HCl (II) with 2-(benzhydryloxy)ethyl chloride (I) in the presence of Na2CO3 and NaI provided the N-alkylated piperidone (III). Horner-Emmons condensation of (III) with triethyl phosphonoacetate (IV) and NaH gave rise to the piperidylidene acetate (V). Then, hydrogenation of the double bond of (V) using Raney Nickel yielded the target compound. |
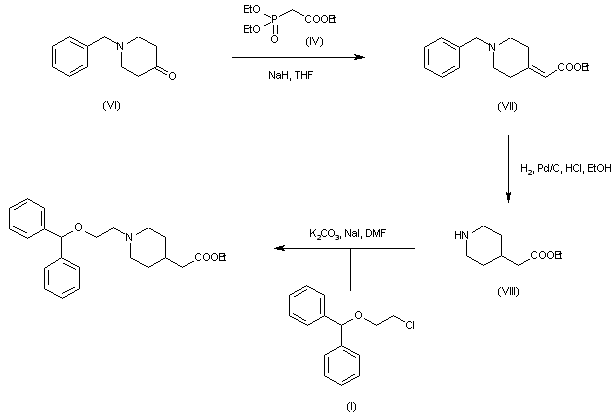 |
| 合成路线图解说明: Horner-Emmons condensation of 1-benzyl-4-piperidone (VI) with phosphonate (IV) furnished the unsaturated ester (VII). Hydrogenation of (VII) over Pd/C produced double bond reduction and simultaneous N-benzyl group cleavage to afford piperidine (VIII). This was finally alkylated with chloride (I) in the presence of K2CO3 and NaI. |
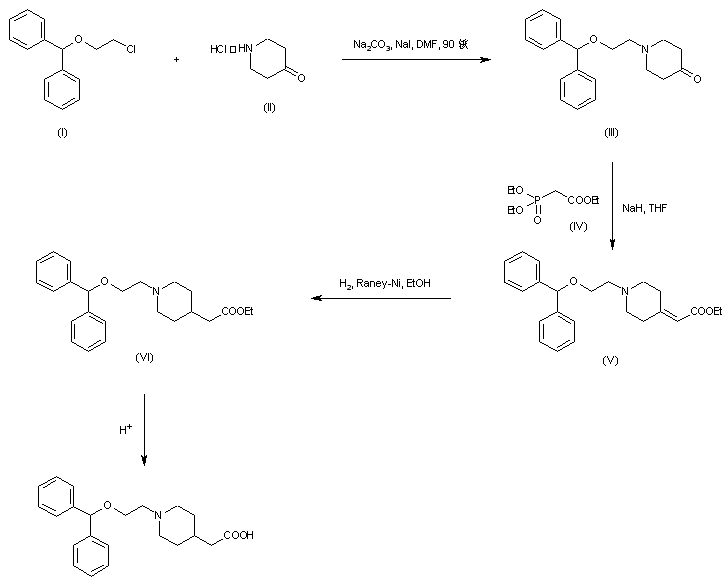 |
| 合成路线图解说明: The title compound was obtained by two related procedures. Reaction of 4-piperidone-HCl (II) with 2-(benzhydryloxy)ethyl chloride (I) in the presence of Na2CO3 and NaI provided the N-alkylated piperidone (III). Horner-Emmons condensation of (III) with triethyl phosphonoacetate (IV) and NaH gave rise to the piperidylidene acetate (V). Then, hydrogenation of the double bond of (V) using Raney Nickel and hydrolysis yielded the target compound. |
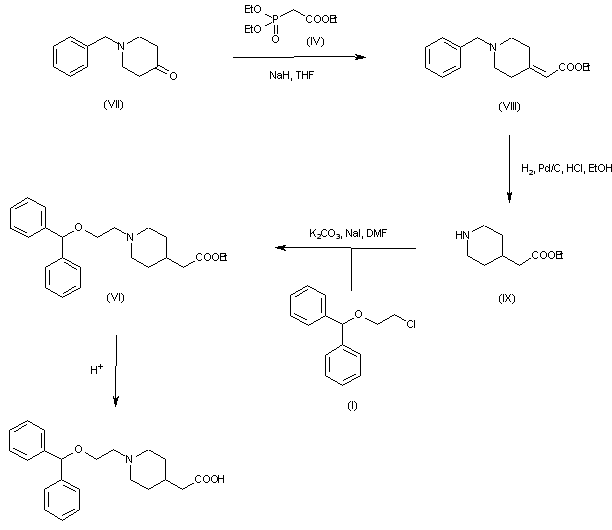 |
| 合成路线图解说明: Horner-Emmons condensation of 1-benzyl-4-piperidone (VII) with phosphonate (IV) furnished the unsaturated ester (VIII). Hydrogenation of (VIII) over Pd/C produced double bond reduction and simultaneous N-benzyl group cleavage to afford piperidine (IX). This was finally alkylated with chloride (I) in the presence of K2CO3 and NaI and hydrolyzed to target compound. |