Grape Seeds Oligomeric Proanthocyanidins
DEFINITION
Grape Seeds Oligomeric Proanthocyanidins is a fraction of an extract of the ripe seeds of Vitis vinifera L. (Fam. Vitaceae). It contains NLT 75.0% of oligomeric proanthocyanidins, on the anhydrous basis. The extract is prepared using suitable solvents such as alcohol, methanol, acetone, ethyl acetate, water, or mixtures of these solvents, in a ratio of starting plant material to extract between 70:1 and 10:1. The extract is further enriched in oligomeric proanthocyanidins by fractionation with ethyl acetate or by other means.
IDENTIFICATION
• A. Thin-Layer Chromatographic Identification Test  201
201
Adsorbent:
Chromatographic silica gel with an average particle size of 5 µm and a layer thickness of about 0.2 mm (HPTLC plates)
Standard solution A:
Dissolve a quantity of USP Purified Grape Seeds Oligomeric Proanthocyanidins RS in methanol, using sonication, to obtain a solution having a concentration of about 5 mg/mL. Centrifuge if necessary, and use the clear supernatant. [Note—Prepare fresh. ]
Standard solution B:
Dissolve a quantity of USP (+)-Catechin RS in methanol, using sonication, to obtain a solution having a concentration of about 1 mg/mL.
Sample solution:
Proceed as directed for Standard solution A, except use the Grape Seeds Oligomeric Proanthocyanidins.
Developing solvent system:
A mixture of acetone, toluene, and formic acid (15:15:5)
Spray reagent:
Dissolve about 100 mg of vanillin in 3-mL of methanol using sonication. Add about 3 mL of hydrochloric acid, dilute with methanol to 10 mL, and carefully mix under cold water. [Note—Prepare fresh. ]
Application volume:
15 µL, as 5–10-mm bands
Analysis
Samples:
Standard solution A, Standard solution B, and Sample solution
Apply the Samples as bands to a suitable thin-layer chromatographic plate (see Chromatography  621
621 ). Use a saturated chamber. Develop the chromatograms until the solvent front has moved up about 90% of the plate. Remove the plate from the chamber, dry, spray with the Spray reagent, dry, and examine under visible light.
). Use a saturated chamber. Develop the chromatograms until the solvent front has moved up about 90% of the plate. Remove the plate from the chamber, dry, spray with the Spray reagent, dry, and examine under visible light.
Acceptance criteria:
The chromatogram of the Sample solution exhibits pink-violet bands, corresponding in color and RF to those in the chromatogram of Standard solution A, at the following approximate RF values: a pair of bands between 0.20 and 0.23 (trimeric proanthocyanidins), a band at 0.28 (proanthocyanidin-B2-3¢-O-gallate), a band at 0.31 (B-type dimeric proanthocyanidins), and a band at 0.43 (( )-epicatechin-3-O-gallate). The chromatogram of the Sample solution may exhibit a pink-violet band at an approximate RF of 0.49 (residual flavan 3-ol monomers and/or gallic acid) corresponding to the band in the chromatogram of Standard solution B. Other pink-violet bands may also be observed.
)-epicatechin-3-O-gallate). The chromatogram of the Sample solution may exhibit a pink-violet band at an approximate RF of 0.49 (residual flavan 3-ol monomers and/or gallic acid) corresponding to the band in the chromatogram of Standard solution B. Other pink-violet bands may also be observed.
• B.
The chromatogram of the Sample solution obtained in the test for Limit of Catechin and Epicatechin exhibits peaks due to proanthocyanidin dimer B1, proanthocyanidin dimer B2, ( )-epicatechin-3-O-gallate, and a broad peak due to other oligomeric proanthocyanidins at retention times corresponding to those in the chromatogram of Standard solution B.
)-epicatechin-3-O-gallate, and a broad peak due to other oligomeric proanthocyanidins at retention times corresponding to those in the chromatogram of Standard solution B.
COMPOSITION
• Content of Oligomeric Proanthocyanidins
Internal standard solution:
Prepare a solution of butylated hydroxytoluene in Mobile phase containing about 0.3 mg/mL.
Standard solution A:
Dissolve a weighed quantity of USP Purified Grape Seeds Oligomeric Proanthocyanidins RS in Internal standard solution to obtain a solution having a known concentration of about 1.0 mg/mL.
Standard solution B:
Dissolve a portion of USP (+)-Catechin RS in Internal standard solution to obtain a solution having a known concentration of about 0.2 mg/mL.
Sample solution:
Dissolve a weighed quantity of Grape Seeds Oligomeric Proanthocyanidins in Internal standard solution to obtain a solution having a known concentration of about 1.0 mg/mL. Centrifuge, and use the clear supernatant.
Mobile phase:
Prepare a filtered and degassed mixture of tetrahydrofuran and an aqueous solution of lithium bromide (about 1 mg/mL) (95:5).
Chromatographic system
Mode:
LC
Detector:
UV 280 nm
Column:
7.5-mm × 30-cm; 5-µm, 500-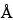 , packing L21
, packing L21
Column temperature:
25 ± 1
± 1
Flow rate:
1.0 mL/min
Injection size:
10 µL
System suitability
Samples:
Standard solution A and Standard solution B
Suitability requirements:
Measure the responses as determined under Analysis.
Relative standard deviation:
NMT 2.0% determined from the the peak area ratio of the oligomeric proanthocyanidins to the internal standard in repeated injections, Standard solution A
Resolution:
NLT 3.0 between the peaks of monomers and the internal standard, Standard solution B
Analysis
Samples:
Standard solution A, Standard solution B, and Sample solution
Chromatograph Standard solution A and determine the beginning and end of the retention time window for the integration of oligomeric proanthocyanidins, at the points where the response of the main peak is about 0.5% of its maximum. Record the peak area ratio of the oligomeric proanthocyanidins to the internal standard. Chromatograph Standard solution B and the Sample solution and identify the locus for the monomers. Integrate the areas of the main peaks within the retention time window as determined for Standard solution A, excluding the area above the main peak, at the locus identified for the monomers, using a proper integration method.
Calculate the percentage of the oligomeric proanthocyanidins in the portion of the Grape Seeds Oligomeric Proanthocyanidins taken:
Result = (RU/RS) × (CS/CU) × 100
| RU | = | = peak response ratio of the oligomeric proanthocyanidins to the internal standard from the Sample solution |
| RS | = | = peak response ratio of the oligomeric proanthocyanidins to the internal standard from Standard solution A |
| CS | = | = concentration of USP Purified Grape Seeds Oligomeric Proanthocyanidins RS in Standard solution A (mg/mL) |
| CU | = | = concentration of Grape Seeds Oligomeric Proanthocyanidins in the Sample solution (mg/mL) |
Acceptance criteria:
NLT 75.0% on the anhydrous basis
IMPURITIES
Inorganic Impurities
• Heavy Metals, Method II  231
231 :
NMT 10 ppm
:
NMT 10 ppm
Organic Impurities
• Articles of Botanical Origin, General Method for Pesticide Residues Analysis  561
561 :
Meets the requirements
:
Meets the requirements
SPECIFIC TESTS
• Limit of Catechin and Epicatechin
Solution A:
Use acetonitrile.
Solution B:
Use a 0.3% aqueous solution of 85% phosphoric acid.
Solvent:
Prepare a mixture of Solution A and Solution B (1:9).
Standard solution A:
Dissolve, using sonication, a weighed quantity of USP (+)-Catechin RS in Solvent to obtain a solution having a known concentration of about 0.5 mg/mL.
Standard solution B:
Dissolve, using sonication, a weighed quantity of USP Grape Seeds Oligomeric Proanthocyanidins RS in Solvent to obtain a solution having a known concentration of about 5 mg/mL. Centrifuge, and use the clear supernatant.
Sample solution:
Proceed as directed for Standard solution B, except use the Grape Seeds Oligomeric Proanthocyanidins.
Mobile phase:
See the gradient table below.
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 10 | 90 |
| 45 | 20 | 80 |
| 65 | 60 | 40 |
| 66 | 10 | 90 |
| 85 | 10 | 90 |
Chromatographic system
Mode:
LC
Detector:
UV 278 nm
Column:
4.6-mm × 25-cm; 5-µm packing L1
Flow rate:
0.7 mL/min
Injection size:
10 µL
System suitability
Samples:
Standard solution A and Standard solution B
Suitability requirements
The chromatogram obtained from Standard solution B is similar to the Reference Chromatogram provided with the lot of the USP Grape Seeds Oligomeric Proanthocyanidins RS being used.
Tailing factor:
NMT 2.0 for the catechin peak, Standard solution A
Relative standard deviation:
NMT 2% determined from the catechin peak in repeated injections, Standard solution A
Analysis
Samples:
Standard solution A, Standard solution B, and Sample solution
Using the chromatogram of Standard solution A, Standard solution B, and the Reference Chromatogram provided with the lot of USP Grape Seeds Oligomeric Proanthocyanidins RS being used, identify the retention times of the peaks corresponding to (+)-catechin and ( )-epicatechin. The approximate relative retention times of the peaks are 1.0 and 1.43 for (+)-catechin and (
)-epicatechin. The approximate relative retention times of the peaks are 1.0 and 1.43 for (+)-catechin and ( )-epicatechin, respectively.
)-epicatechin, respectively.
Calculate the sum of the percentages of (+)-catechin and ( )-epicatechin in the portion of the Grape Seeds Oligomeric Proanthocyanidins taken:
)-epicatechin in the portion of the Grape Seeds Oligomeric Proanthocyanidins taken:
(rU/rS) × (C × V/W) × 100
| rU | = | = sum of the peak responses for (+)-catechin and ( |
| rS | = | = peak response for (+)-catechin in Standard solution A |
| CS | = | = concentration of USP (+)-Catechin RS in Standard solution A (mg/mL) |
| V | = | = final volume of the Sample solution (mL) |
| W | = | = weight of Grape Seeds Oligomeric Proanthocyanidins taken to prepare the Sample solution (mg) |
Acceptance criteria:
NMT 19.0% on the anhydrous basis
• Microbial Enumeration Tests  2021
2021 :
The total aerobic microbial count does not exceed 104 cfu/g. The total combined yeast and mold count does not exceed 103 cfu/g.
:
The total aerobic microbial count does not exceed 104 cfu/g. The total combined yeast and mold count does not exceed 103 cfu/g.
• Absence of Specified Microorganisms  2022
2022 :
It meets the requirements of the tests for absence of Salmonella species and Escherichia coli.
:
It meets the requirements of the tests for absence of Salmonella species and Escherichia coli.
• Residue on Ignition  281
281 :
NMT 0.5%, determined on 5.0 g
:
NMT 0.5%, determined on 5.0 g
• Water, Method Ia  921
921 :
NMT 8.0%
:
NMT 8.0%
• Water-Insoluble Fraction
Analysis:
Transfer about 1 g, weighed, to a suitable flask. Add 100 mL of water, and shake vigorously for 15 min. Pass the solution through a previously tared sintered-glass filter, wash the flask with 30 mL of water, and transfer the washings to the filter. Wash the filter with 30 mL of water in 5-mL portions. Dry the filter for 2 h at 105 , cool in a desiccator, and weigh. Calculate the percentage of the water-insoluble fraction.
, cool in a desiccator, and weigh. Calculate the percentage of the water-insoluble fraction.
Acceptance criteria:
NMT 2%
• Other Requirements:
It meets the requirements of the test for Residual Solvents under Botanical Extracts  565
565 .
.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers, protected from light and moisture, and store at controlled room temperature.
• Labeling:
The label states the Latin binomial and, following the official name, it states “Grape Seeds Oligomeric Proanthocyanidins”. It meets other labeling requirements under Botanical Extracts  565
565 .
.
• USP Reference Standards  11
11
USP Grape Seeds Oligomeric Proanthocyanidins RS
USP Purified Grape Seeds Oligomeric Proanthocyanidins RS
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Maged H. Sharaf, Ph.D.
Principal Scientific Liaison 1-301-816-8318 |
(DS2010) Monographs - Dietary Supplements |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1347
Pharmacopeial Forum: Volume No. 34(3) Page 659
