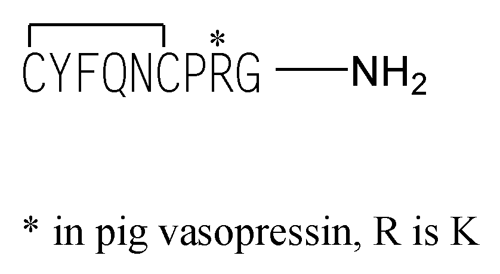Vasopressin
(vay'' soe pres' in).
DEFINITION
Change to read:
Vasopressin is a polypeptide hormone having the properties of causing the contraction of vascular and other smooth muscles, and of antidiuresis. It is prepared by chemical synthesis. It contains NLT 95.0% and NMT  105.0%
105.0% (RB 1-Jul-2011) of vasopressin (C46H65N15O12S2), calculated on the anhydrous, acetic acid-free basis.
(RB 1-Jul-2011) of vasopressin (C46H65N15O12S2), calculated on the anhydrous, acetic acid-free basis.
IDENTIFICATION
• A.
The retention time of the vasopressin peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
• B. Mass Spectral Analysis
Infusion solution:
Acetonitrile, water, and trifluoroacetic acid (80: 20: 0.08)
Standard solution:
1 mg/mL of USP Vasopressin RS in water
Sample solution:
1 mg/mL of Vasopressin in water.
[Note—The final concentrations of the Standard solution and the Sample solution can be adjusted, depending on the sensitivity of the mass spectrometer used in the testing. ]
Instrumental conditions
(See Mass Spectrometry  736
736 .)
.)
Mode:
LC/MS spectrometer
Interface/detection:
Infusion system connected to an electrospray interface (positive ion)
Flow rate:
0.3 mL/min
Injection size:
10 µL
Analysis
Samples:
Standard solution and Sample solution
Acceptance criteria:
Should contain peaks with mass-to-charge ratios of 1084 and 543
ASSAY
Change to read:
• Procedure
Mobile phase:
Dissolve 6.6 g of dibasic ammonium phosphate in 950 mL of water. Adjust with concentrated phosphoric acid to a pH of 3.0. Dilute with water to 1000 mL. To 870 mL of this solution add 130 mL of acetonitrile, and mix. Filter under vacuum through a nylon membrane of 0.45-µm pore size. [Note—The retention time of the vasopressin peak is very sensitive to small changes in acetonitrile concentration in the Mobile phase. ]
System suitability solution:
Dissolve suitable quantities of USP Lypressin RS and USP Vasopressin RS in 0.25% glacial acetic acid to obtain a solution having a known concentration of about 25 µg/mL of each substance.
Standard solution:
Dissolve the entire contents of a vial of USP Vasopressin RS in a known volume of 0.25% glacial acetic acid. [Note—The solution may be diluted as necessary to a working concentration range for the Assay. ]
Sample solution:
Transfer about 10 mg of Vasopressin to a 25-mL volumetric flask. Dissolve in 0.25% glacial acetic acid, and dilute with the same solvent to volume.
Chromatographic system
Mode:
LC
Detector:
UV 220 nm
Column:
4.6-mm × 25-cm; packing L1
Column temperature:
40 ± 1
Flow rate:
1.0 mL/min
Injection size:
20 µL. [Note—The column is allowed to equilibrate for 1 h before making the first injection. ]
System suitability
Samples:
System suitability solution and Standard solution. [Note—Inject into an equilibrated liquid chromatograph, allowing about 60 min for complete elution. ]
[Note—The retention time of the vasopressin peak is between 6 and 9 min. ]
Suitability requirements
Resolution:
NLT 1.1 between the vasopressin and lypressin peaks
Relative standard deviation:
NMT 2.0% for the vasopressin peak
Analysis
Samples:
Standard solution and Sample solution
Calculate the  percentage of vasopressin (C46H65N15O12S2) in the portion of Vasopressin taken:
percentage of vasopressin (C46H65N15O12S2) in the portion of Vasopressin taken: (RB 1-Jul-2011)
(RB 1-Jul-2011)
Result =  (rU/rS) × (CS/CU) × 100
(rU/rS) × (CS/CU) × 100 (RB 1-Jul-2011)
(RB 1-Jul-2011)
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| = | = concentration of USP Vasopressin RS in the Standard solution (mg/mL) | |
| CU | = | = concentration of Vasopressin in the Sample solution (mg/mL) |
Acceptance criteria:
95.0%– 105.0%
105.0% (RB 1-Jul-2011) on the anhydrous, acetic acid-free basis
(RB 1-Jul-2011) on the anhydrous, acetic acid-free basis
IMPURITIES
• Ordinary Impurities:
The sum of the responses of impurities from the Sample solution in the Assay is NMT 5% of the area of the vasopressin peak.
SPECIFIC TESTS
• Microbial Enumeration Tests  61
61 and Tests for Specified Microorganisms
and Tests for Specified Microorganisms  62
62 :
The total bacterial count is NMT 2 × 102 cfu/g. For products of animal origin, it also meets the requirements of the tests for absence of Salmonella species and Escherichia coli.
:
The total bacterial count is NMT 2 × 102 cfu/g. For products of animal origin, it also meets the requirements of the tests for absence of Salmonella species and Escherichia coli.
• Water Determination, Method Ic  921
921 :
NMT 8.0%
:
NMT 8.0%
• Acetic Acid in Peptides  503
503 :
NMT 15.0%
:
NMT 15.0%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers, preferably of Type I glass, and store in a refrigerator.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Thomas A. Sigambris, M.S.
Scientific Liaison 1-301-998-6789 |
(BIO12010) Monographs - Biologics and Biotechnology 1 |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 5005
Pharmacopeial Forum: Volume No. 34(4) Page 994