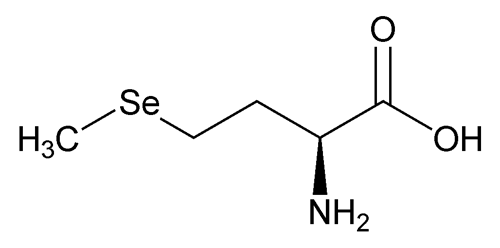
Selenomethionine
C5H11NO2Se 196.11
Butanoic acid, 2-amino-4-(methylseleno)-, (S)-;
(S)-2-Amino-4-(methylselenyl)butyric acid

 [1464-42-2].
[1464-42-2].
(se lee'' noe me thye' oh neen).
C5H11NO2Se 196.11
Butanoic acid, 2-amino-4-(methylseleno)-, (S)-;
(S)-2-Amino-4-(methylselenyl)butyric acid
DEFINITION
Selenomethionine contains NLT 97.0% and NMT 103.0% of selenomethionine (C5H11NO2Se) and contains NLT 39.0% and NMT 41.0% of selenium (Se), calculated on the as-is basis.
IDENTIFICATION
ASSAY
• Procedure
Mobile phase:
6.8 g/L of monobasic potassium phosphate in water. Adjust with phosphoric acid to a pH of 2.75 ± 0.25.
System suitability solution:
0.16 mg/mL of USP Selenomethionine RS and 0.8 mg/mL of USP l-Methionine RS in Mobile phase
Standard solution:
0.16 mg/mL of USP Selenomethionine RS in Mobile phase
Sample solution:
0.16 mg/mL of Selenomethionine in Mobile phase with sonication. Pass through a membrane filter of 0.45-µm pore size.
Chromatographic system
Mode:
LC
Detector:
UV 220 nm
Column:
4.6-mm × 25-cm; packing L1 with polar end-capping
Flow rate:
1 mL/min
Injection size:
20 µL
System suitability
Sample:
System suitability solution
[Note—The relative retention times for methionine and selenomethionine are 0.8 and 1.0, respectively. ]
Suitability requirements
Resolution:
NLT 3.0 between methionine and selenomethionine
Tailing factor:
NMT 2.0 for the selenomethionine peak
Relative standard deviation:
NMT 2.0% for the selenomethionine peak
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of selenomethionine (C5H11NO2Se) in the portion of Selenomethionine taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Selenomethionine RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Selenomethionine in the Sample solution (mg/mL) |
Acceptance criteria:
97.0%–103.0% on the as-is basis
IMPURITIES
• Heavy Metals, Method I  231
231 :
NMT 20 ppm
:
NMT 20 ppm
• Limit of Sodium
Standard stock solution:
10 µg/mL of sodium from sodium chloride, previously dried at 105 for 2 h
for 2 h
Standard solutions:
0.2, 0.5, and 1.0 µg/mL of sodium. Pipet 2.0, 5.0, and 10.0 mL of Standard stock solution into separate 100-mL volumetric flasks. To each flask add 2.0 mL of potassium chloride solution (1 in 5) and 1.0 mL of hydrochloric acid, and dilute with water to volume.
Sample solution:
Transfer 100 mg of Selenomethionine to a 100-mL volumetric flask, add 2.0 mL of potassium chloride solution (1 in 5) and 1.0 mL of hydrochloric acid, and dilute with water to volume.
Instrumental conditions
Mode:
Atomic absorption spectrophotometry
Analytical wavelength:
589 nm
Lamp:
Sodium hollow-cathode
Flame:
Air–acetylene
Analysis
Samples:
Standard solutions and Sample solution
Determine the absorbances of the solutions. Plot the absorbances of the Standard solutions versus their concentrations (µg/mL of sodium), and draw the straight line best fitting the plotted points. From the graph so obtained, determine the concentration of sodium, CNa (µg/mL), in the Sample solution.
Calculate the percentage of sodium in the portion of Selenomethionine taken:
Result = (CNa/CSA) × F × 100
| CNa | = | = concentration of sodium in the Sample solution (µg/mL), determined from the regression line |
| CSA | = | = concentration of Selenomethionine in the Sample solution (mg/mL) |
| F | = | = conversion factor, 0.001 mg/µg |
Acceptance criteria:
NMT 0.1%
• Chromatographic Purity
Standard solution A:
Transfer 50 mg of USP Selenomethionine RS to a 10-mL volumetric flask, add 2 mL of water, warm to dissolve if necessary, and dilute with methanol to volume.
Standard solution B:
Transfer 1.0 mL of Standard solution A to a 100-mL volumetric flask, and dilute with methanol to volume.
Sample solution:
Transfer 50 mg of Selenomethionine to a 10-mL volumetric flask, add 2 mL of water, warm to dissolve if necessary, and dilute with methanol to volume.
Chromatographic system
(See Chromatography  621
621 , System Suitability.)
, System Suitability.)
Mode:
TLC
Absorbent:
0.25-mm layer of chromatographic silica gel mixture
Application volume:
10 µL
Developing solvent:
Butanol, glacial acetic acid, and water (4:1:1)
Spray reagent:
2 mg/mL of ninhydrin in alcohol
Analysis
Samples:
Standard solution A, Standard solution B, and Sample solution
Proceed as directed for Chromatography  621
621 , Thin-Layer Chromatography. Allow the spots to dry, and develop the chromatogram in the Developing solvent until the solvent front has moved three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and allow the solvent to evaporate. Locate the spots on the plate by spraying with Spray reagent and drying it at 110
, Thin-Layer Chromatography. Allow the spots to dry, and develop the chromatogram in the Developing solvent until the solvent front has moved three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and allow the solvent to evaporate. Locate the spots on the plate by spraying with Spray reagent and drying it at 110 for 10 min.
for 10 min.
Acceptance criteria:
NMT 1.0%. The RF value of the principal spot of the Sample solution corresponds to that of Standard solution A; and no spot, other than the principal spot of the Sample solution, is larger or more intense than the principal spot of Standard solution B.
SPECIFIC TESTS
• Optical Rotation, Specific Rotation  781S
781S
Sample solution:
10 mg/mL in 1 N hydrochloric acid
Acceptance criteria:
+17.0 to +19.5
to +19.5
• Content of Selenium
[Caution—Selenium is toxic; handle it with care.
]
Standard stock solution:
Dissolve 1 g of metallic selenium in a minimum volume of nitric acid. Evaporate to dryness, add 2 mL of water, and evaporate to dryness. Repeat the addition of water and evaporation to dryness three times. Dissolve the residue in 3 N hydrochloric acid, transfer to a 1000-mL volumetric flask, and dilute with 3 N hydrochloric acid to volume. This solution contains 1000 µg/mL of selenium.
Standard solutions:
20, 50, and 100 µg/mL of selenium. Pipet 2.0, 5.0, and 10.0 mL of Standard stock solution into separate 100-mL volumetric flasks. Dilute the contents of each flask with water to volume.
Sample solution:
0.125 mg/mL of Selenomethionine in water
Instrumental conditions
Mode:
Atomic absorption spectrophotometry
Analytical wavelength:
196 nm
Lamp:
Selenium hollow-cathode
Flame:
Air–acetylene
Blank:
Water
Analysis
Samples:
Standard solutions and Sample solution
Determine the absorbances of the solutions. Plot the absorbances of the Standard solutions versus their concentrations (µg/mL of selenium), and draw the straight line best fitting the plotted points. From the graph so obtained, determine the concentration of selenium, CSe (µg/mL), in the Sample solution.
Calculate the percentage of selenium in the portion of Selenomethionine taken:
Result = (CSe/CSA) × F × 100
| CSe | = | = concentration of selenium in the Sample solution (µg/mL), determined from the regression line |
| CSA | = | = concentration of Selenomethionine in the Sample solution (mg/mL) |
| F | = | = conversion factor, 0.001 mg/µg |
Acceptance criteria:
39.0%–41.0% on the as-is basis
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Huy T. Dinh, M.S.
Scientific Liaison 1-301-816-8594 |
(DS2010) Monographs - Dietary Supplements |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1444
Pharmacopeial Forum: Volume No. 31(2) Page 482