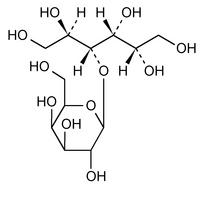
Lactitol
C12H24O11·H2O 362.34
C12H24O11·2H2O 380.35
4-O-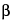 -d-Galactopyranosyl-d-glucitol
-d-Galactopyranosyl-d-glucitol


 [585-86-4].
[585-86-4].
Monohydrate

 [81025-04-9].
[81025-04-9].
Dihydrate

 [81025-03-8].
[81025-03-8].
(lak' ti tol).
C12H24O11·H2O 362.34
C12H24O11·2H2O 380.35
4-O-
Monohydrate
Dihydrate
DEFINITION
Lactitol contains NLT 98.0% and NMT 101.0% of C12H24O11, calculated on the anhydrous basis.
IDENTIFICATION
ASSAY
• Procedure
Mobile phase:
Water
Standard solution:
10.0 mg/mL of USP Lactitol RS
Sample solution:
10.0 mg/mL of Lactitol
Chromatographic system
Mode:
LC
Detector:
Refractive index
Column:
7.8-mm × 30-cm; packing L34
Column temperature:
85
Flow rate:
0.7 mL/min
Injection size:
25 µL
System suitability
Sample:
Standard solution
Suitability requirements
Relative standard deviation:
NMT 1.0% for lactitol
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of lactitol (C12H24O11) in the portion of Lactitol taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Lactitol RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Lactitol in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–101.0% on the anhydrous basis
IMPURITIES
• Residue on Ignition  281
281 :
NMT 0.5%
:
NMT 0.5%
• Related Compounds
Standard solution:
0.3 mg/mL of USP Lactitol RS
Sample solution:
Prepare as directed in the Assay.
Chromatographic system:
Proceed as directed in the Assay.
System suitability
Sample:
Standard solution
[Note—The relative retention times for lactose, glucose, galactose, lactulitol, lactitol, galactitol, and sorbitol are about 0.53, 0.58, 0.67, 0.72, 1.0, 1.55, and 1.68, respectively. ]
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentages of galactitol, sorbitol, lactulitol, lactose, glucose, and galactose in the portion of Lactitol taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of the relevant related compound, if observed, from the Sample solution |
| rS | = | = peak response of lactitol from the Standard solution |
| CS | = | = concentration of USP Lactitol RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Lactitol in the Sample solution (mg/mL) |
Acceptance criteria:
The total of the percentages of all related compounds is NMT 1.5%.
• Reducing Sugars
Standard solution:
Pipet 2 mL of a dextrose solution containing 0.5 mg/mL into a 10-mL conical flask.
Sample solution:
Disolve 500 mg in 2.0 mL of water in a 10-mL conical flask.
Analysis:
Concomitantly add 1 mL of alkaline cupric tartrate TS to each solution, heat to boiling, and cool.
Acceptance criteria:
NMT 0.2%, calculated as dextrose. The Sample solution shows no more turbidity than that produced in the Standard solution, in which a reddish brown precipitate forms.
SPECIFIC TESTS
• Water Determination, Method I  921
921 :
For the monohydrate form, 4.5%–5.5%; for the dihydrate form, 9.5%–10.5%; and for the anhydrous form, NMT 0.5%.
:
For the monohydrate form, 4.5%–5.5%; for the dihydrate form, 9.5%–10.5%; and for the anhydrous form, NMT 0.5%.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers.
• Labeling:
Label it to indicate whether it is the monohydrate, the dihydrate, or the anhydrous form.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S.
Scientific Liaison 1-301-816-8335 |
(EXC2010) Monographs - Excipients |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1836
Pharmacopeial Forum: Volume No. 31(4) Page 1143