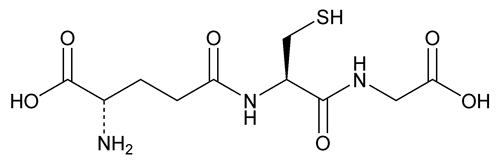
Glutathione
Pentanoic acid, 2-amino-5-[(R)-1-(carboxymethylamino)-3-mercapto-1-oxopropan-2-ylamino]-5-oxo, (S);
N-(N-l-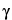 -Glutamyl-l-cysteinyl)glycine
-Glutamyl-l-cysteinyl)glycine


 [70-18-8].
[70-18-8].
Pentanoic acid, 2-amino-5-[(R)-1-(carboxymethylamino)-3-mercapto-1-oxopropan-2-ylamino]-5-oxo, (S);
N-(N-l-
DEFINITION
Glutathione contains NLT 98.0% and NMT 101.0% of C10H17N3O6S, as glutathione, calculated on the dried basis.
IDENTIFICATION
• B. Optical Rotation, Specific Rotation  781S
781S
Sample solution:
40 mg/mL in water
Acceptance criteria:
 15.5
15.5 to
to  17.5
17.5 , at 20
, at 20
ASSAY
• Procedure
Sample:
500 mg of glutathione previously dried
Titrimetric system
(See Titrimetry  541
541 .)
.)
Mode:
Direct titration
Titrant:
0.1 N iodine VS
Endpoint detection:
Visual
Blank:
50 mL of metaphosphoric acid (1 in 50)
Analysis:
Dissolve the Sample in 50 mL of metaphosphoric acid (1 in 50) and titrate with the Titrant.
Calculate the percentage of glutathione (C10H17N3O6S) in the portion of Glutathione taken:
Result = [(V  B) × N × F × 100]/W
B) × N × F × 100]/W
| V | = | = titrant volume of the Sample (mL) |
| B | = | = titrant volume of the Blank (mL) |
| N | = | = titrant normality (mEq/mL) |
| F | = | = equivalency factor, 307.32 mg/mEq |
| W | = | = weight of the Sample (mg) |
Acceptance criteria:
98.0%–101.0% on the dried basis
IMPURITIES
Inorganic Impurities
• Ammonium
Standard solution:
10 µg of ammonium from a diluted ammonium chloride solution
Sample solution:
50 mg of Glutathione
Analysis:
Transfer the Sample solution and the Standard solution to separate 25-mL jars fitted with caps, and dissolve in 1 mL of water. Add 0.30 g of magnesium oxide. Close immediately after placing a piece of silver manganese paper 5-mm square, wetted with a few drops of water, under the caps. Swirl, avoiding projections of liquid, and allow to stand at 40 for 30 min.
for 30 min.
Acceptance criteria:
If the silver manganese paper shows a gray color, it is not more intense than the standard (NMT 200 ppm).
• Arsenic  211
211 :
NMT 2 ppm
:
NMT 2 ppm
• Chloride and Sulfate, Chloride  221
221 :
Dissolve 0.7 g with water to make 15 mL. The solution shows no more chloride than corresponds to 0.20 mL of 0.020 N hydrochloric acid (NMT 200 ppm).
:
Dissolve 0.7 g with water to make 15 mL. The solution shows no more chloride than corresponds to 0.20 mL of 0.020 N hydrochloric acid (NMT 200 ppm).
• Chloride and Sulfate, Sulfate  221
221 :
Dissolve 0.8 g in water to make 15 mL. The solution shows no more sulfate than corresponds to 0.25 mL of 0.020 N sulfuric acid (NMT 300 ppm).
:
Dissolve 0.8 g in water to make 15 mL. The solution shows no more sulfate than corresponds to 0.25 mL of 0.020 N sulfuric acid (NMT 300 ppm).
• Heavy Metals, Method I  231
231 :
NMT 10 ppm
:
NMT 10 ppm
• Iron  241
241 :
NMT 10 ppm
:
NMT 10 ppm
• Residue on Ignition  281
281 :
NMT 0.1%
:
NMT 0.1%
Organic Impurities
• Procedure
Mobile phase:
6.8 g/L of potassium dihydrogen phosphate with 2.02 g/L of sodium 1-heptane sulfonate. Adjust with phosphoric acid to a pH of 3.0. Mix 970 mL of this solution with 30 mL of methanol.
System suitability solution:
0.1 mg/mL of USP l-Phenylalanine RS, 0.5 mg/mL of USP Glutathione RS, and 0.5 mg/mL of USP Ascorbic acid RS in Mobile phase
Standard solution:
0.01 mg/mL of USP Glutathione RS in Mobile phase. [Note—This solution has a concentration equivalent to 2.0% of that of the Sample solution. ]
Sample solution:
50 mg of glutathione in 100 mL of Mobile phase. [Note—Allow the solution to stand for 5 min before use. ]
Chromatographic system
Mode:
LC
Detector:
UV 210 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Column temperature:
30
Flow rate:
Adjust so that the retention time of glutathione is about 5 min.
Injection size:
10 µL
System suitability
Sample:
System suitability solution
Suitability requirements
Resolution:
NLT 5.0 between the ascorbic acid and glutathione peaks; and NLT 5.0 between the glutathione and l-phenylalanine peaks
Relative standard deviation:
NMT 1.5% for replicate injections
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of any impurity in the portion of Glutathione taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of any peak from the Sample solution other than glutathione |
| rS | = | = peak response of the glutathione peak from the Standard solution |
| CS | = | = concentration of USP Glutathione RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Glutathione in the Sample solution (mg/mL) |
Acceptance criteria
Individual impurity:
NMT 1.5% for the impurity with the relative retention time of about 4
Total impurities:
NMT 2.0%
SPECIFIC TESTS
• Clarity and Color of Solution
Sample solution:
0.1 g/mL in water
Analysis:
Using identical tubes of colorless, transparent, neutral glass with a flat base and an internal diameter of 15–25 mm, compare the liquid to be examined with water, the depth of the layer being 40 mm. Compare the colors in diffused daylight, viewing vertically against a white background.
Acceptance criteria:
The solution is clear and colorless.
• Loss on Drying  731
731 :
NMT 0.5%, 105
:
NMT 0.5%, 105 for 3 h
for 3 h
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Huy T. Dinh, M.S.
Scientific Liaison 1-301-816-8594 |
(DS2010) Monographs - Dietary Supplements |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1342
Pharmacopeial Forum: Volume No. 36(5) Page 1221