Change to read:
Naphthalene,
1,3-Naphthalenediol (Naphthoresorcinol),
Solubility in methanol—
Dissolve 500 mg in 50 mL of methanol: the solution is clear and complete.
2,7-Naphthalenediol (2,7-Dihydroxynaphthalene),
2-Naphthalenesulfonic Acid,
Assay—
Dissolve about 1 g, accurately weighed, in 100 mL of water, add phenolphthalein TS, and titrate with 0.1 N sodium hydroxide VS. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N sodium hydroxide is equivalent to 22.63 mg of C10H8O3S·H2O. Not less than 98.0% is found.
Melting range  741
741 :
between 122
:
between 122 and 126
and 126 , but the range between beginning and end of melting does not exceed 2
, but the range between beginning and end of melting does not exceed 2 .
.
1-Naphthol (Alphanaphthol),
2-Naphthol (Betanaphthol),
Solubility in alcohol—
A solution of 1 g in 10 mL of alcohol is complete and colorless or practically so.
Residue on ignition (Reagent test):
not more than 0.05%.
Acidity—
Shake 1 g with 50 mL of water occasionally during 15 minutes, and filter: the filtrate is neutral to litmus.
1-Naphthol—
Boil 100 mg with 10 mL of water until dissolved, cool, and filter. Add to the filtrate 0.3 mL of 1 N sodium hydroxide and 0.3 mL of 0.1 N iodine: no violet color is produced.
Insoluble in ammonia (naphthalene, etc.)—
Shake 500 mg with 30 mL of ammonia TS: the 2-naphthol dissolves completely and the solution is not darker than pale yellow.
p-Naphtholbenzein,
Naphthol Dipotassium Disulfonate (2-Naphthol-6,8-dipotassium Disulfonate),
[note—A suitable grade is available as “2-naphthyl-6,8-disulfonic acid dipotassium salt” from Pfaltz and Bauer, Inc., www.pfaltzandbauer.com.]
Naphthol Disodium Disulfonate (2-Naphthol-3,6-disodium Disulfonate),
Loss on drying  731
731 —
Dry it in vacuum at about 50
—
Dry it in vacuum at about 50 : it loses not more than 2.0% of its weight.
: it loses not more than 2.0% of its weight.
Residue on ignition (Reagent test)—
Ignite 1 g of dried sample with 3 mL of sulfuric acid: the residue weighs between 265 and 280 mg (between 26.5% and 28.0%).
Naphthoresorcinol (1,3-Dihydroresorcinol),
1-Naphthylamine,
1-Naphthylamine Hydrochloride,
A 1 in 100 solution, make slightly acid with acetic acid, gives a violet color with 5 drops of ferric chloride TS. A 1 in 40 solution in diluted acetic acid is colorless and not more than slightly opalescent.
Residue on ignition (Reagent test)—
Ignite 200 mg with a few drops of sulfuric acid: the weight of the residue is negligible.
2-Naphthyl Chloroformate (Chloroformic Acid 2-Naphthyl Ester),
N-(1-Naphthyl)ethylenediamine Dihydrochloride,
Neutralized Alcohol —See Alcohol, Neutralized.
Nickel,
Nickel-Aluminum Catalyst —Use a suitable grade.
[note—A suitable grade is “Raney Nickel, Active Catalyst,” available as “aluminum–nickel alloy,” catalog number 72240, available from Fluka Chemical Corp., fax 1-800-962-9591, Web site: www.sigma-aldrich.com.]
Nickel Sulfate,
Nickel (II) Sulfate Heptahydrate,
Assay—
Dissolve 17.9 g of anhydrous dibasic sodium phosphate in water to make 500 mL (Solution A). Dissolve 6.8 g of monobasic potassium phosphate in water to make 500 mL (Solution B). To a volume of Solution A, add Solution B until the mixture is adjusted to a pH of 7.0 (about 2:1 by volume of Solutions A and B) to obtain a pH 7.0 Buffer. Transfer about 25 mg of 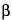 -nicotinamide adenine dinucleotide, accurately weighed, to a 25-mL volumetric flask, dissolve in and dilute with water to volume, and mix. Transfer 0.2 mL of this solution to a 10-mL volumetric flask, dilute with pH 7.0 Buffer to volume, and mix. Use this solution as the Assay preparation. Determine the absorbances of the Assay preparation and the pH 7.0 Buffer in 1-cm cells at a wavelength of 260 nm, using water as the reference. Calculate the quantity, in mg, of C21H27N7O14P2 in the portion of
-nicotinamide adenine dinucleotide, accurately weighed, to a 25-mL volumetric flask, dissolve in and dilute with water to volume, and mix. Transfer 0.2 mL of this solution to a 10-mL volumetric flask, dilute with pH 7.0 Buffer to volume, and mix. Use this solution as the Assay preparation. Determine the absorbances of the Assay preparation and the pH 7.0 Buffer in 1-cm cells at a wavelength of 260 nm, using water as the reference. Calculate the quantity, in mg, of C21H27N7O14P2 in the portion of 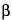 -nicotinamide adenine dinucleotide taken by the formula:
-nicotinamide adenine dinucleotide taken by the formula:
(0.6634/17.6)(10/0.2)(25)(AA – AB)
in which AA and AB are the absorbances of the Assay preparation and the pH 7.0 Buffer, respectively. Not less than 94.5% is found.
Nicotinamide Adenine Dinucleotide Phosphate-adenosine-5¢-triphosphate Mixture —Use a suitable grade.
Suitability—
When used in the assay of lactulose, determine that a suitable absorbance-versus-concentration slope is obtained, using USP Lactulose RS , the reagent blank absorbance being not more than 0.020. The commercially available reagent contains 64 mg of nicotinamide adenine dinucleotide phosphate and 160 mg of adenosine-5¢-triphosphate per vial. The mixture is buffered and stabilized. For use in the Assay of lactulose it is diluted with water to 100 mL.
Nicotinic Acid —Use Niacin (USP monograph).
Ninhydrin,
Nitric Acid,
Nitric Acid, Diluted
Nitric Acid, Fuming (90 Percent Nitric Acid),
Nitric Acid, Lead-Free— Use ACS reagent grade.
Lead—
To 100 g add 0.1 g of anhydrous sodium carbonate and evaporate to dryness. Dissolve the residue in water, heating slightly, and dilute with the same solvent to 50.0 mL. Determine the lead content by atomic absorption spectrophotometry (see Spectrophotometry and Light-Scattering  851
851 ) measuring the absorbance at 283.3 nm or 217.0 nm using a lead hollow-cathode lamp and an air–acetylene flame. It contains not more than 0.1 ppm of lead (Pb).
) measuring the absorbance at 283.3 nm or 217.0 nm using a lead hollow-cathode lamp and an air–acetylene flame. It contains not more than 0.1 ppm of lead (Pb).
Nitric Oxide–Nitrogen Dioxide Detector Tube— A fuse-sealed glass tube so designed that gas may be passed through it and containing suitable absorbing filters and support media for an oxidizing layer and the indicator diphenyl benzidine.
Measuring range:
0.5 to 10 ppm.
[note—Available from Draeger Safety, Inc., www.draeger.com, or from Gastec Corp., www.gastec.co.jp, distributed in the USA by www.nextteq.com.]
Nitrilotriacetic Acid,
4¢-Nitroacetophenone (p¢-Nitroacetophenone),
Assay—
Inject an appropriate ether solution of the specimen (about 0.5 µL) into a suitable gas chromatograph (see Chromatography 621
621 ) equipped with a thermal conductivity detector, helium being used as the carrier gas. The following conditions have been found suitable: a 4-mm × 1.8-m stainless steel column containing 10% phase G1 on support S1A; the injection port and detector temperatures are maintained at 200
) equipped with a thermal conductivity detector, helium being used as the carrier gas. The following conditions have been found suitable: a 4-mm × 1.8-m stainless steel column containing 10% phase G1 on support S1A; the injection port and detector temperatures are maintained at 200 and 300
and 300 , respectively; the column temperature is maintained at 170
, respectively; the column temperature is maintained at 170 and programmed to rise 3
and programmed to rise 3 per minute to 220
per minute to 220 . The area of the 4¢-nitroacetophenone peak is not less than 97% of the total peak area.
. The area of the 4¢-nitroacetophenone peak is not less than 97% of the total peak area.
o-Nitroaniline,
p-Nitroaniline,
Solubility—
Separate 1-g portions dissolve in 30 mL of alcohol and in 40 mL of ether, respectively, to yield solutions that are clear or practically so.
Residue on ignition (Reagent test):
not more than 0.2%.
Nitrobenzene,
p-Nitrobenzenediazonium Tetrafluoroborate,
Assay—
Transfer about 30 mg, accurately weighed, to a low-actinic, 100-mL volumetric flask. Dissolve in 0.01 N hydrochloric acid, dilute with 0.01 N hydrochloric acid to volume, and mix. Using low-actinic glassware, dilute 2.0 mL of the resulting solution with spectrophotometric grade methanol to 50.0 mL. Measure the absorbance of this solution in a 1-cm cell at about 255 nm, using methanol as the blank. Calculate the absorptivity of the solution by dividing the measured absorbance by the concentration in g per mL. Calculate the assay value by the formula:
100a / 59.4
in which a is the absorptivity of the solution: not less than 95.0% is found.
p-Nitrobenzyl Bromide,
Solubility—
Separate 200-mg portions yield clear solutions in 5 mL of alcohol and in 5 mL of glacial acetic acid.
Residue on ignition (Reagent test):
 negligible, from 200 mg.
negligible, from 200 mg.
Change to read:
4-(p-Nitrobenzyl)pyridine,
Nitromethane,
5-Nitro-1,10-phenanthroline,
Suitability as redox indicator—
Dissolve 25 mg in a minimum volume of diluted sulfuric acid, add 10 mg of ferrous sulfate, and dilute with water to 100 mL: the solution is deep red in color and exhibits an absorption maximum at 510 nm. To 1.0 mL of the solution add 1.0 mL of 0.01 M ceric sulfate: the red color is discharged.
1-Nitroso-2-naphthol,
Assay—
Transfer about 250 mg, previously dried over silica gel to constant weight and accurately weighed, to a glass-stoppered flask, and dissolve in 10 mL of sodium hydroxide solution (1 in 10). Cool the solution in an ice bath, add dilute sulfuric acid (1 in 6) until a slight, permanent precipitate is formed and the solution is slightly acid, then add 3 g of potassium iodide, shake to dissolve, add 20 mL of dilute sulfuric acid (1 in 6), immediately insert the stopper in the flask, and allow to stand in the dark for 2 hours. Titrate the liberated iodine with 0.1 N sodium thiosulfate VS, adding 3 mL of starch TS as the endpoint is approached. Perform a complete blank determination, and make any necessary correction. Each mL of 0.1 N sodium thiosulfate is equivalent to 8.66 mg of C10H7NO2: not less than 95.0% is found.
Residue on ignition (Reagent test):
not more than 0.2%.
Nitroso R Salt (1-Nitroso-2-naphthol-3,6-disodium Disulfonate),
Sensitiveness—
Dissolve 500 mg of sodium acetate in a solution of 0.4 mg of cobaltous chloride (0.1 mg of cobalt) in 5 mL of water. Add 1 mL of diluted acetic acid, and follow with 1 mL of a solution of the nitroso R salt (1 in 500): a red color, which is produced at once, persists when the solution is boiled with 1 mL of hydrochloric acid for 1 minute.
Nitrous Oxide Certified Standard
Nonadecane,
Assay—
Inject an appropriate specimen into a suitable gas chromatograph (see Chromatography  621
621 ) equipped with a thermal conductivity detector, helium being used as the carrier gas. The following conditions have been found suitable: 3-mm × 1.8-m stainless steel column containing 5% phase G2 on support S1AB; the injection port temperature is maintained at 330
) equipped with a thermal conductivity detector, helium being used as the carrier gas. The following conditions have been found suitable: 3-mm × 1.8-m stainless steel column containing 5% phase G2 on support S1AB; the injection port temperature is maintained at 330 ; the detector temperature is maintained at 300
; the detector temperature is maintained at 300 ; and the oven temperature is held initially at 190
; and the oven temperature is held initially at 190 and allowed to rise gradually to 250
and allowed to rise gradually to 250 . The area of the nonadecane peak is not less than 99% of the total peak area.
. The area of the nonadecane peak is not less than 99% of the total peak area.
Nonanoic Acid,
Assay—
Accurately weigh about 500 mg, transfer to a suitable container, add 30 mL of water, and mix. Add 40 mL of water, and mix. Add phenolphthalein TS, and titrate with 0.1 N sodium hydroxide VS. Each mL of 0.1 N sodium hydroxide is equivalent to 15.82 mg of C9H18O2: not less than 96.0% of C9H18O2 is found.
Refractive index  831
831 :
about 1.432 at 20
:
about 1.432 at 20 .
.
Nonionic Wetting Agent— Use a suitable amphoteric surfactant.
[note—A suitable grade is commercially available as Triton X-100 or Octoxynol 9.]
1-Nonyl Alcohol (1-Nonanol),
Assay—
Not less than 97% of C9H20O is found, a suitable gas chromatograph equipped with a flame-ionization detector and helium being used as the carrier gas at a flow rate of about 40 mL per minute. The following conditions have been found suitable: a 3.2-mm × 1.83-m stainless steel column packed with 20% phase G16 on support S1A; the injection port, column, and detector temperatures are maintained at about 250 , 160
, 160 , and 310
, and 310 , respectively.
, respectively.
n-Nonylamine (1-Aminononane),
Nonylphenol Polyoxyethylene Ether,
Nonylphenoxypoly(ethyleneoxy)ethanol —Clear, viscous, pale yellow liquid. May exhibit slight solidification on cooling; warming with agitation will restore to original condition. Density: about 1.06. Soluble in alcohol, in xylene, and in water. Suitable for use in gas–liquid chromatography.
[note—A suitable grade is “Igepal CO 710,” available from General Aniline and Film Corp., 140 West 51st St., New York, NY 10020.]
Normal Butyl Alcohol —See Butyl Alcohol.
Normal Butyl Nitrite —See n-Butyl Nitrite.
Normal Butylamine —See n-Butylamine.