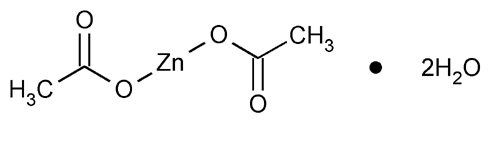
Zinc Acetate
Acetic acid, zinc salt, dihydrate.
Zinc acetate dihydrate
Anhydrous 183.48
» Zinc Acetate contains not less than 98.0 percent and not more than 102.0 percent of C4H6O4Zn·2H2O.
Packaging and storage—
Preserve in tight containers.
pH  791
791 :
between 6.0 and 8.0, in a solution (1 in 20).
:
between 6.0 and 8.0, in a solution (1 in 20).
Insoluble matter—
A 20-g portion, dissolved in 150 mL of water containing 1 mL of glacial acetic acid, shows not more than 1.0 mg of insoluble matter (0.005%).
Arsenic, Method I  211
211 :
3 ppm.
:
3 ppm.
Lead  251
251 —
Dissolve 0.5 g in 1 mL of a mixture of equal parts, by volume, of nitric acid and water in a separator. Add 3 mL of Ammonium Citrate Solution and 0.5 mL of Hydroxylamine Hydrochloride Solution, and render alkaline, with ammonium hydroxide, to phenol red TS. Add 10 mL of Potassium Cyanide Solution, and immediately extract the solution with successive 5-mL portions of Dithizone Extraction Solution, draining off each extract into another separator, until the last portion of dithizone solution retains its green color. Shake the combined extracts for 30 seconds with 20 mL of dilute nitric acid (1 in 100), and discard the chloroform layer. Add to the acid solution 4.0 mL of Ammonia-Cyanide Solution and 2 drops of Hydroxylamine Hydrochloride Solution. Add 10.0 mL of Standard Dithizone Solution, and shake the mixture for 30 seconds. Filter the chloroform layer through acid-washed filter paper into a color-comparison tube, and compare the color with that of a standard prepared as follows: to 20 mL of dilute nitric acid (1 in 100) add 0.01 mg of lead, 4 mL of Ammonia-Cyanide Solution, and 2 drops of Hydroxylamine Hydrochloride Solution, and shake for 30 seconds with 10.0 mL of Standard Dithizone Solution. Filter through acid-washed filter paper into a color-comparison tube: the color of the sample solution does not exceed that of the control (0.002%).
—
Dissolve 0.5 g in 1 mL of a mixture of equal parts, by volume, of nitric acid and water in a separator. Add 3 mL of Ammonium Citrate Solution and 0.5 mL of Hydroxylamine Hydrochloride Solution, and render alkaline, with ammonium hydroxide, to phenol red TS. Add 10 mL of Potassium Cyanide Solution, and immediately extract the solution with successive 5-mL portions of Dithizone Extraction Solution, draining off each extract into another separator, until the last portion of dithizone solution retains its green color. Shake the combined extracts for 30 seconds with 20 mL of dilute nitric acid (1 in 100), and discard the chloroform layer. Add to the acid solution 4.0 mL of Ammonia-Cyanide Solution and 2 drops of Hydroxylamine Hydrochloride Solution. Add 10.0 mL of Standard Dithizone Solution, and shake the mixture for 30 seconds. Filter the chloroform layer through acid-washed filter paper into a color-comparison tube, and compare the color with that of a standard prepared as follows: to 20 mL of dilute nitric acid (1 in 100) add 0.01 mg of lead, 4 mL of Ammonia-Cyanide Solution, and 2 drops of Hydroxylamine Hydrochloride Solution, and shake for 30 seconds with 10.0 mL of Standard Dithizone Solution. Filter through acid-washed filter paper into a color-comparison tube: the color of the sample solution does not exceed that of the control (0.002%).
Chloride  221
221 —
A 1.5-g portion shows no more chloride than corresponds to 0.10 mL of 0.020 N hydrochloric acid (0.005%).
—
A 1.5-g portion shows no more chloride than corresponds to 0.10 mL of 0.020 N hydrochloric acid (0.005%).
Sulfate  221
221 —
A 1.0-g portion shows no more sulfate than corresponds to 0.10 mL of 0.020 N sulfuric acid (0.010%).
—
A 1.0-g portion shows no more sulfate than corresponds to 0.10 mL of 0.020 N sulfuric acid (0.010%).
Alkalies and alkaline earths—
Dissolve 2.0 g in about 150 mL of water contained in a 200-mL volumetric flask, add sufficient ammonium sulfide TS to precipitate the zinc completely, dilute with water to volume, and mix. Filter through a dry filter, rejecting the first portion of the filtrate. To 100 mL of the subsequent filtrate add 5 drops of sulfuric acid, evaporate to dryness, and ignite: the weight of the residue does not exceed 2 mg (0.2%).
Assay—
Dissolve about 400 mg of Zinc Acetate, accurately weighed, in 100 mL of water. Add 5 mL of ammonia–ammonium chloride buffer TS and 0.1 mL of eriochrome black TS, and titrate with 0.05 M edetate disodium VS until the solution is deep blue in color. Each mL of 0.05 M edetate disodium is equivalent to 10.98 mg of C4H6O4Zn·2H2O.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Sujatha Ramakrishna, Ph.D.
Scientist 1-301-816-8349 |
(MDCV05) Monograph Development-Cardiovascular |
USP32–NF27 Page 3893
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.