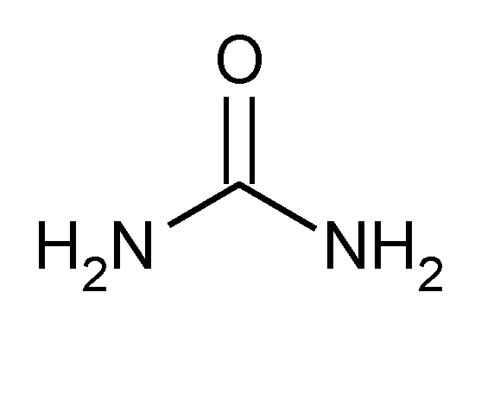
Urea
» Urea contains not less than 99.0 percent and not more than 100.5 percent of CH4N2O.
Packaging and storage—
Preserve in well-closed containers. Store at 25 , excursions permitted between 15
, excursions permitted between 15 and 30
and 30 .
.
Labeling—
Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
Identification—
A:
Heat about 500 mg in a test tube: it liquefies, and ammonia is evolved. Continue the heating until the liquid becomes turbid, then cool. Dissolve the fused mass in a mixture of 10 mL of water and 1 mL of sodium hydroxide solution (1 in 10), and add 1 drop of cupric sulfate TS: the solution acquires a reddish-violet color.
B:
Dissolve 100 mg in 1 mL of water, and add 1 mL of nitric acid: a white crystalline precipitate of urea nitrate is formed.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Alcohol-insoluble matter—
Dissolve 5.0 g in 50 mL of warm alcohol, and if any insoluble residue remains, filter the solution on a tared filter, wash the residue and the filter with 20 mL of warm alcohol, and dry at 105 for 1 hour: the weight of the residue does not exceed 2 mg (0.04%).
for 1 hour: the weight of the residue does not exceed 2 mg (0.04%).
Chloride  221
221 —
A 2.0-g portion shows no more chloride than corresponds to 0.20 mL of 0.020 N hydrochloric acid (0.007%).
—
A 2.0-g portion shows no more chloride than corresponds to 0.20 mL of 0.020 N hydrochloric acid (0.007%).
Sulfate  221
221 —
A 2.0-g portion shows no more sulfate than corresponds to 0.20 mL of 0.020 N sulfuric acid (0.010%).
—
A 2.0-g portion shows no more sulfate than corresponds to 0.20 mL of 0.020 N sulfuric acid (0.010%).
Heavy metals  231
231 —
Dissolve 1.0 g in 20 mL of water, and add 5 mL of 0.1 N hydrochloric acid: the limit is 0.002%.
—
Dissolve 1.0 g in 20 mL of water, and add 5 mL of 0.1 N hydrochloric acid: the limit is 0.002%.
Other requirements—
Where the label states that Urea is sterile, it meets the requirements for Sterility Tests  71
71 and for Bacterial endotoxins under Urea for Injection. Where the label states that Urea must be subjected to further processing during the preparation of injectable dosage forms, it meets the requirements for Bacterial endotoxins under Urea for Injection.
and for Bacterial endotoxins under Urea for Injection. Where the label states that Urea must be subjected to further processing during the preparation of injectable dosage forms, it meets the requirements for Bacterial endotoxins under Urea for Injection.
Assay—
Transfer about 500 mg of Urea, accurately weighed, to a 200-mL volumetric flask, dissolve in and dilute with water to volume, and mix. Pipet 2 mL of this solution into a micro-Kjeldahl digestion flask, and proceed as directed under Nitrogen Determination, Method II  461
461 , beginning with “Add 1 g of a powdered mixture.” [note—In this procedure, continue heating the flask until fuming begins, then heat for 1 additional hour.] Each mL of 0.01 N acid is equivalent to 0.3003 mg of CH4N2O.
, beginning with “Add 1 g of a powdered mixture.” [note—In this procedure, continue heating the flask until fuming begins, then heat for 1 additional hour.] Each mL of 0.01 N acid is equivalent to 0.3003 mg of CH4N2O.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Sujatha Ramakrishna, Ph.D.
Scientist 1-301-816-8349 |
(MDCV05) Monograph Development-Cardiovascular |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 3831
Pharmacopeial Forum: Volume No. 29(5) Page 1589