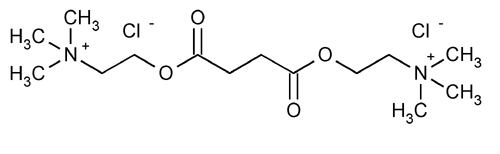
Succinylcholine Chloride
Ethanaminium, 2,2¢-[(1,4-dioxo-1,4-butanediyl)bis(oxy)]bis[N,N,N-trimethyl]-, dichloride.
Choline chloride succinate (2:1)
Dihydrate 397.34
» Succinylcholine Chloride usually contains approximately two molecules of water of hydration. It contains not less than 96.0 percent and not more than 102.0 percent of C14H30Cl2N2O4, calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers. Store at 25 , excursions permitted between 15
, excursions permitted between 15 and 30
and 30 .
.
Labeling—
Label it in terms of its anhydrous equivalent. Where it is intended for use in preparing injectable or other sterile dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
USP Reference standards  11
11 —
—
USP Choline Chloride RS.
USP Endotoxin RS.
USP Succinylcholine Chloride RS.
USP Succinylmonocholine Chloride RS.
USP Choline Chloride RS.
USP Endotoxin RS.
USP Succinylcholine Chloride RS.
USP Succinylmonocholine Chloride RS.
Identification—
B:
The retention time of the major peak in the chromatogram of the Assay preparation correspond to that in the chromatogram of the Standard preparation, as obtained in the Assay.
C:
Dissolve a portion in water to obtain a solution containing 1 mg per mL. Applying 1-µL portions to a plate coated with a 0.25-mm layer of chromatographic silica gel (see Chromatography  621
621 ), and using a solvent system consisting of a mixture of acetone and 1 N hydrochloric acid (1:1), proceed as directed under Thin-Layer Chromatographic Identification Test
), and using a solvent system consisting of a mixture of acetone and 1 N hydrochloric acid (1:1), proceed as directed under Thin-Layer Chromatographic Identification Test  201
201 . Use the following procedure to locate the spots. Heat the plate at 105
. Use the following procedure to locate the spots. Heat the plate at 105 for 5 minutes, cool, and spray with potassium bismuth iodide TS, then heat again at 105
for 5 minutes, cool, and spray with potassium bismuth iodide TS, then heat again at 105 for 5 minutes.
for 5 minutes.
Water, Method I  921
921 :
not more than 10.0%.
:
not more than 10.0%.
Residue on ignition  281
281 :
not more than 0.2%.
:
not more than 0.2%.
Chromatographic purity—
test 1—
Buffer solution—
Prepare a solution in water containing 3.85 g per L of 1-pentanesulfonic acid, 2.9 g per L of sodium chloride, and 1% (v/v) of 1 N sulfuric acid.
Mobile phase—
Prepare a filtered and degassed mixture of Buffer solution and acetonitrile (95:5).
System suitability solution—
Dissolve accurately weighed quantities of citric acid and succinic acid in Mobile phase to obtain a solution containing about 0.5 mg of each per mL.
Standard solution—
Dissolve an accurately weighed quantity of USP Succinylmonocholine Chloride RS in Mobile phase, and dilute quantitatively, and stepwise if necessary, with Mobile phase to obtain a solution having a known concentration of about 0.05 mg per mL.
Test solution—
Transfer about 100 mg of Succinylcholine Chloride, accurately weighed, to a 10-mL volumetric flask, and dissolve in and dilute with Mobile phase to volume.
Chromatographic system (see Chromatography  621
621 )—
The chromatograph is equipped with a 214-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L1. The flow rate is about 1 mL per minute. Samples are maintained at a temperature of about 4
)—
The chromatograph is equipped with a 214-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L1. The flow rate is about 1 mL per minute. Samples are maintained at a temperature of about 4 during the analysis. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the resolution, R, between citric acid and succinic acid is not less than 2.9. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 3.0%.
during the analysis. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the resolution, R, between citric acid and succinic acid is not less than 2.9. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 3.0%.
Procedure—
Separately inject equal volumes (about 50 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the peak responses. Begin integration after the edetate disodium peak, if present (retention time is about 3.5 minutes). The relative retention times are about 0.22 for succinic acid, 0.32 for the doublet of peaks quantitated as a single component, 0.49 for succinylmonocholine chloride, and 1.0 for succinylcholine chloride. Calculate the percentage of each impurity in the portion of Succinylcholine Chloride taken by the formula:
10C(ri / rS)F
in which C is the concentration, in mg per mL, of USP Succinylmonocholine Chloride RS in the Standard solution; ri is the peak area for each impurity obtained from the Test solution; rS is the succinylmonocholine chloride peak area obtained from the Standard solution; and F is the response factor (0.63 for succinic acid): not more than 0.1% of succinic acid is found; not more than 0.4% of the doublet of peaks quantitated as a single component is found; not more than 0.4% of succinylmonocholine chloride is found; and not more than 0.2% of any other individual impurity is found.
test 2 (limit of choline)—
Solution A—
Prepare a solution in water containing 5% (v/v) of acetonitrile and 5% (w/v) of 0.1 M 1-hexanesulfonic acid.
Solution B—
Prepare a solution of acetonitrile and water (1:1).
Mobile phase—
Use variable amounts of Solution A and Solution B as directed for Chromatographic system. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
System suitability solution—
Dissolve an accurately weighed quantity of USP Choline Chloride RS and sodium chloride in water; and dilute quantitatively, and stepwise if necessary, with water to obtain a solution containing 0.05 mg per mL and 0.01 mg per mL, respectively.
Standard stock solution—
Dissolve an accurately weighed quantity of USP Choline Chloride RS in water; and dilute quantitatively, and stepwise if necessary, with water to obtain a solution containing 0.5 mg per mL.
Standard solution—
Dilute 1 mL of the Standard stock solution with water to 50 mL.
Test solution—
Transfer about 50 mg of Succinylcholine Chloride, accurately weighed, to a 25-mL flask, and dissolve in and dilute with water to volume.
Chromatographic system (see Chromatography  621
621 )—
The ion chromatograph is equipped with a suitable device for chemical suppression, a conductivity detector at 30 µS, and a 4.6-mm × 25-cm column that contains 5-µm packing L1. The eluent flow rate is about 1 mL per minute, and uses deionized water at a flow rate of 5–10 mL per minute as the regenerant for the chemical suppressor and a suppressor current setting of 50 mA.
)—
The ion chromatograph is equipped with a suitable device for chemical suppression, a conductivity detector at 30 µS, and a 4.6-mm × 25-cm column that contains 5-µm packing L1. The eluent flow rate is about 1 mL per minute, and uses deionized water at a flow rate of 5–10 mL per minute as the regenerant for the chemical suppressor and a suppressor current setting of 50 mA.
Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the resolution, R, between sodium and choline is not less than 2.0; and the relative standard deviation for replicate injections is not more than 3.0%.
| Time (minutes) |
Solution A
(%) |
Solution B
(%) |
Elution |
| 0–15 | 100 | 0 | isocratic |
| 15–16 | 100®0 | 0®100 | linear gradient |
| 16–25 | 0 | 100 | isocratic |
| 25–27 | 0®100 | 100®0 | linear gradient |
| 27–40 | 100 | 0 | isocratic |
Procedure—
Separately inject equal volumes (about 50 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the peak responses. Calculate the percentage of choline in the portion of Succinylcholine Chloride taken by the formula:
37.5C(rC / rS)
in which C is the concentration, in mg per mL, of USP Choline Chloride RS in the Standard solution; and rC and rS are the choline peak areas obtained from the Test solution and the Standard solution, respectively: not more than 0.3% of choline is found; and not more than 1.5% of total impurities is found, the results for Test 1 and Test 2 being added.
Chloride content—
Dissolve about 400 mg, accurately weighed, in 5 mL of water. Add 5 mL of glacial acetic acid, 50 mL of methanol, and 1 drop of eosin Y TS, and titrate with 0.1 N silver nitrate VS. Each mL of 0.1 N silver nitrate is equivalent to 3.545 mg of Cl. Not less than 19.3% and not more than 19.8% of Cl, calculated on the anhydrous basis, is found.
Other requirements—
Where the label states that Succinylcholine Chloride is sterile, it meets the requirements for Sterility Tests  71
71 and for Bacterial endotoxins under Succinylcholine Chloride for Injection. Where the label states that Succinylcholine Chloride must be subjected to further processing during the preparation of injectable dosage forms, it meets the requirements for Bacterial endotoxins under Succinylcholine Chloride for Injection.
and for Bacterial endotoxins under Succinylcholine Chloride for Injection. Where the label states that Succinylcholine Chloride must be subjected to further processing during the preparation of injectable dosage forms, it meets the requirements for Bacterial endotoxins under Succinylcholine Chloride for Injection.
Assay—
[note—Since the Mobile phase employed in this procedure has a fairly high concentration of chloride ion and a low pH, it is advisable to rinse the entire system with water following the use of this Mobile phase.]
Mobile phase—
Prepare a 1 in 10 solution of 1 N aqueous tetramethylammonium chloride in methanol. Pass this solution through a 0.45-µm membrane filter, and adjust with hydrochloric acid to a pH of about 3.0.
Standard preparation—
Transfer about 88 mg of USP Succinylcholine Chloride RS, accurately weighed, to a 10-mL volumetric flask, add 4.0 mL of water, and dilute with Mobile phase to volume while mixing. Prepare the Standard preparation concurrently with the Assay preparation.
Assay preparation—
Transfer about 88 mg of Succinylcholine Chloride, accurately weighed, to a 10-mL volumetric flask, add 4.0 mL of water, and dilute with Mobile phase to volume while mixing.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 214-nm detector and a 4-mm × 25-cm column that contains packing L3. The flow rate is about 0.75 mL per minute. Chromatograph five replicate injections of the Standard preparation, and record the peak responses as directed for Procedure: the tailing factor is not greater than 2.5; and the relative standard deviation for replicate injections is not more than 1.5%.
)—
The liquid chromatograph is equipped with a 214-nm detector and a 4-mm × 25-cm column that contains packing L3. The flow rate is about 0.75 mL per minute. Chromatograph five replicate injections of the Standard preparation, and record the peak responses as directed for Procedure: the tailing factor is not greater than 2.5; and the relative standard deviation for replicate injections is not more than 1.5%.
Procedure—
Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph by means of a suitable microsyringe or sampling valve, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of C14H30Cl2N2O4 in the Succinylcholine Chloride taken by the formula:
10C(rU / rS)
in which C is the concentration, in mg per mL, of anhydrous succinylcholine chloride in the Standard preparation, as determined from the concentration of USP Succinylcholine Chloride RS corrected for moisture content by a titrimetric water determination; rU is the peak response obtained from the Assay preparation; and rS is the average peak response obtained from the Standard preparation.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Daniel K. Bempong, Ph.D.
Senior Scientist 1-301-816-8143 |
(MDPS05) Monograph Development-Pulmonary and Steroids |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 3604
Pharmacopeial Forum: Volume No. 32(6) Page 1754
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.