Sodium Bicarbonate
NaHCO3




 84.01
84.01
Carbonic acid monosodium salt.
Monosodium carbonate
» Sodium Bicarbonate contains not less than 99.0 percent and not more than 100.5 percent of NaHCO3, calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers.
Labeling—
Where Sodium Bicarbonate is intended for use in hemodialysis, it is so labeled.
Identification—
A solution of it meets the requirements of the tests for Sodium  191
191 and for Bicarbonate
and for Bicarbonate  191
191 .
.
Loss on drying  731
731 —
Dry about 4 g, accurately weighed, over silica gel for 4 hours: it loses not more than 0.25% of its weight.
—
Dry about 4 g, accurately weighed, over silica gel for 4 hours: it loses not more than 0.25% of its weight.
Insoluble substances—
Dissolve 1 g in 20 mL of water: the resulting solution is complete and clear.
Carbonate (where it is labeled as intended for use in hemodialysis)—
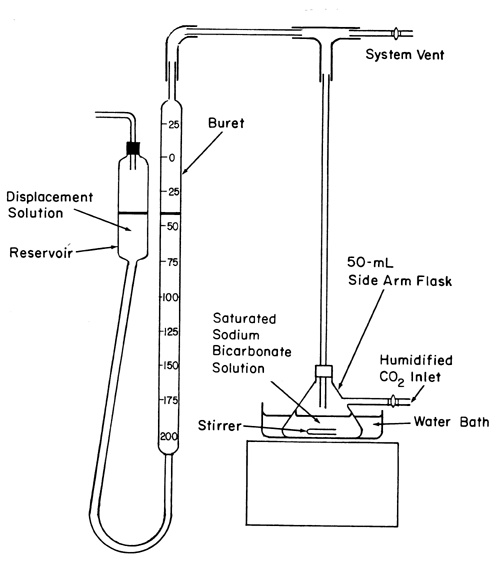
Apparatus—
The apparatus (see illustration)
consists of a 50-mL flask with a side arm connected to a source of carbon dioxide humidified by bubbling through a saturated solution of sodium bicarbonate and equipped with a top-mounted stopper fitted with an exit tube connected via a T-tube to a system vent and a leveling buret and reservoir.
Reagents—
Saturated sodium bicarbonate solution —
Mix about 20 g of sodium bicarbonate and 100 mL of water, and allow any undissolved crystals to settle. Use the clear supernatant.
Displacement solution—
Dissolve 100 g of sodium chloride in 350 mL of water, add about 1 g of sodium bicarbonate and 1 mL of methyl orange TS. After the sodium bicarbonate has dissolved, add 6 N sulfuric acid until the solution turns pink. Use this solution to fill the reservoir of the apparatus.
Procedure—
Add 25 mL of Saturated sodium bicarbonate solution to the 50-mL flask, and flush the system by allowing humidified carbon dioxide to enter through the side arm. Close the carbon dioxide inlet and the system vent, and stir the Saturated sodium bicarbonate solution until no further carbon dioxide absorption is noted from successive buret readings. Maintain atmospheric pressure in the apparatus by adjusting the Displacement solution to the same level in both the reservoir and the buret, noting the buret reading. Open the system vent, and reintroduce humidified carbon dioxide through the side arm of the flask. Close the carbon dioxide inlet and the system vent, and stir the Saturated sodium bicarbonate solution vigorously until no further carbon dioxide absorption is noted. Repeat the carbon dioxide absorption procedure starting with “Open the system vent” until no more than a 0.2-mL change in buret reading is noted. Discontinue stirring, reintroduce humidified carbon dioxide through the side arm of the flask, remove the top-mounted stopper from the flask briefly, and promptly add about 10 g of Sodium Bicarbonate, accurately weighed, to the flask. Replace the stopper, continue the flow of humidified carbon dioxide for about 30 seconds, and then close the carbon dioxide inlet and the system vent. Stir the solution in the flask vigorously until carbon dioxide absorption ceases, noting the volume absorbed from the buret reading. Restore atmospheric pressure in the apparatus by leveling the Displacement solution in the reservoir and the buret, and discontinue stirring. Open the system vent, and flush humidified carbon dioxide through the system. Close the carbon dioxide inlet and the system vent, and stir the solution in the flask vigorously until carbon dioxide absorption ceases. Determine the total volume, V, in mL, of carbon dioxide absorbed after the addition of the specimen to the flask, and calculate the percentage of carbonate in the portion of specimen tested by the formula:
273V(6001P)/[22400(273 + T)(760W)]
in which P is the ambient atmospheric pressure, in mm of mercury, T is the ambient temperature, and W is the quantity, in g, of specimen taken. [note—Maintain a constant temperature during the measurement of the volume of carbon dioxide absorbed.] The limit of carbonate is not more than 0.23%.
Normal carbonate—
Add 2.0 mL of 0.10 N hydrochloric acid and 2 drops of phenolphthalein TS to 1.0 g of Sodium Bicarbonate, previously dissolved with very gentle swirling in 20 mL of water at a temperature not exceeding 15 : the solution does not assume more than a faint pink color immediately.
: the solution does not assume more than a faint pink color immediately.
Chloride  221
221 :
a 0.35-g portion shows no more chloride than corresponds to 1.48 mL of 0.0010 N hydrochloric acid (0.015%).
:
a 0.35-g portion shows no more chloride than corresponds to 1.48 mL of 0.0010 N hydrochloric acid (0.015%).
Limit of sulfur compounds—
Dissolve 2.0 g of Sodium Bicarbonate in 20 mL of water, evaporate to 5 mL by boiling, add 1 mL of bromine TS, evaporate to dryness, and cool. Dissolve the residue in 10 mL of 3 N hydrochloric acid, evaporate to dryness, and cool. Dissolve the residue in 5 mL of 3 N hydrochloric acid, evaporate to dryness, and cool. Dissolve the residue in 10 mL of water, and adjust with 3 N hydrochloric acid or 6 N ammonium hydroxide to a pH of 2. If necessary to obtain a clear solution, filter the solution, washing the filter with two 2-mL portions of water. Dilute with water to 20 mL (test solution). To a 0.30 mL of 0.020 N sulfuric acid, add 1 mL of 0.06 N hydrochloric acid, and dilute with water to 20 mL (Standard solution). Add 1 mL of barium chloride TS to the test solution and the Standard solution, mix, and allow to stand for 30 minutes. Any turbidity produced in the test solution is not more intense than that produced in the Standard solution: not more than 0.015% is found.
Aluminum  206
206 (where it is labeled as intended for use in hemodialysis)—
Proceed as directed except to prepare the Test Preparation as follows. Transfer 1.0 g of Sodium Bicarbonate to a 100-mL plastic volumetric flask, and carefully add 4 mL of nitric acid. Sonicate for 30 minutes, dilute with water to volume, and mix. The limit is 2 µg per g.
(where it is labeled as intended for use in hemodialysis)—
Proceed as directed except to prepare the Test Preparation as follows. Transfer 1.0 g of Sodium Bicarbonate to a 100-mL plastic volumetric flask, and carefully add 4 mL of nitric acid. Sonicate for 30 minutes, dilute with water to volume, and mix. The limit is 2 µg per g.
Arsenic, Method I  211
211 —
Prepare the Test Preparation by dissolving 1.5 g in 20 mL of 7 N sulfuric acid, and adding 35 mL of water: the resulting solution meets the requirements of the test, the addition of 20 mL of 7 N sulfuric acid specified under Procedure being omitted. The limit is 2 µg per g.
—
Prepare the Test Preparation by dissolving 1.5 g in 20 mL of 7 N sulfuric acid, and adding 35 mL of water: the resulting solution meets the requirements of the test, the addition of 20 mL of 7 N sulfuric acid specified under Procedure being omitted. The limit is 2 µg per g.
Calcium and magnesium (where it is labeled as intended for use in hemodialysis)—
[note—The Standard preparations and the Test preparation may be modified, if necessary, to obtain solutions, of suitable concentrations, adaptable to the linear or working range of the instrument.]
Potassium chloride solution—
Dissolve 10 g of potassium chloride in 1000 mL of 0.36 N hydrochloric acid.
Calcium standard preparations—
Transfer 249.7 mg of calcium carbonate, previously dried at 300 for 3 hours and cooled in a desiccator for 2 hours, to a 100-mL volumetric flask. Dissolve in 6 mL of 6 N hydrochloric acid, add 1 g of potassium chloride, dilute with water to volume, and mix. Transfer 10.0 mL of this solution to a second 100-mL volumetric flask, dilute with Potassium chloride solution to volume, and mix. This solution contains 100 µg of Ca per mL. Transfer 2.0-, 3.0-, 4.0-, and 5.0-mL portions of this solution to separate 100-mL volumetric flasks (each containing 6 mL of 6 N hydrochloric acid), dilute with Potassium chloride solution to volume, and mix. These Calcium standard preparations contain 2.0, 3.0, 4.0, and 5.0 µg of Ca per mL, respectively.
for 3 hours and cooled in a desiccator for 2 hours, to a 100-mL volumetric flask. Dissolve in 6 mL of 6 N hydrochloric acid, add 1 g of potassium chloride, dilute with water to volume, and mix. Transfer 10.0 mL of this solution to a second 100-mL volumetric flask, dilute with Potassium chloride solution to volume, and mix. This solution contains 100 µg of Ca per mL. Transfer 2.0-, 3.0-, 4.0-, and 5.0-mL portions of this solution to separate 100-mL volumetric flasks (each containing 6 mL of 6 N hydrochloric acid), dilute with Potassium chloride solution to volume, and mix. These Calcium standard preparations contain 2.0, 3.0, 4.0, and 5.0 µg of Ca per mL, respectively.
Magnesium standard preparations—
Place 1.000 g of magnesium in a 250-mL beaker containing 20 mL of water, and carefully add 20 mL of hydrochloric acid, warming if necessary to complete the reaction. Transfer this solution to a 1000-mL volumetric flask containing 10 g of potassium chloride, dilute with water to volume, and mix. Transfer 10.0 mL of this solution to a 100-mL volumetric flask containing 1 g of potassium chloride, dilute with water to volume, and mix. Transfer 10.0 mL of this solution to a second 100-mL volumetric flask, dilute with Potassium chloride solution to volume, and mix. This solution contains 10.0 µg of Mg per mL. Transfer 2.0-, 3.0-, 4.0-, and 5.0-mL portions of this solution to separate 100-mL volumetric flasks (each containing 6 mL of 6 N hydrochloric acid), dilute with Potassium chloride solution to volume, and mix. These Magnesium standard preparations contain 0.2, 0.3, 0.4, and 0.5 µg of Mg per mL, respectively.
Test preparation—
Transfer 3.0 g of Sodium Bicarbonate to a 100-mL volumetric flask, add 6 mL of 6 N hydrochloric acid and 1 g of potassium chloride, dilute with water to volume, and mix.
Procedure for calcium—
Concomitantly determine the absorbances of the Calcium standard preparations and the Test preparation at the calcium emission line at 422.7 nm with a suitable atomic absorption spectrophotometer (see Spectrophotometry and Light-Scattering  851
851 ) equipped with a calcium hollow-cathode lamp and a nitrous oxide–acetylene flame, using Potassium chloride solution as the blank. Plot the absorbances of the Calcium standard preparations versus their contents of calcium, in µg per mL, by drawing a straight line best fitting the four plotted points. From the graph so obtained determine the quantity, in µg, of Ca in each mL of the Test preparation. Calculate the percentage of Ca in the specimen taken by dividing this value by 300: the limit is 0.01%.
) equipped with a calcium hollow-cathode lamp and a nitrous oxide–acetylene flame, using Potassium chloride solution as the blank. Plot the absorbances of the Calcium standard preparations versus their contents of calcium, in µg per mL, by drawing a straight line best fitting the four plotted points. From the graph so obtained determine the quantity, in µg, of Ca in each mL of the Test preparation. Calculate the percentage of Ca in the specimen taken by dividing this value by 300: the limit is 0.01%.
Procedure for magnesium—
Concomitantly determine the absorbances of the Magnesium standard preparations and the Test preparation at the magnesium emission line at 285.2 nm with a suitable atomic absorption spectrophotometer (see Spectrophotometry and Light-Scattering  851
851 ) equipped with a magnesium hollow-cathode lamp and a reducing air–acetylene flame, using Potassium chloride solution as the blank. Plot the absorbances of the Magnesium standard preparations versus their contents of magnesium, in µg per mL, by drawing a straight line best fitting the four plotted points. From the graph so obtained determine the quantity, in µg, of Mg in each mL of the Test preparation. Calculate the percentage of Mg in the specimen taken by dividing this value by 300: the limit is 0.004%.
) equipped with a magnesium hollow-cathode lamp and a reducing air–acetylene flame, using Potassium chloride solution as the blank. Plot the absorbances of the Magnesium standard preparations versus their contents of magnesium, in µg per mL, by drawing a straight line best fitting the four plotted points. From the graph so obtained determine the quantity, in µg, of Mg in each mL of the Test preparation. Calculate the percentage of Mg in the specimen taken by dividing this value by 300: the limit is 0.004%.
Copper (where it is labeled as intended for use in hemodialysis)—
[note—The Standard preparation and the Test preparation may be modified, if necessary, to obtain solutions, of suitable concentrations, adaptable to the linear or working range of the instrument.]
Nitric acid diluent—
Dilute 40 mL of nitric acid to 1000 mL with water.
Standard preparation—
Transfer 1.000 g of copper to a 1000-mL volumetric flask, dissolve in 20 mL of nitric acid, dilute with 0.2 N nitric acid to volume, and mix. Transfer 10.0 mL of this solution to a second 1000-mL volumetric flask, dilute with 0.2 N nitric acid to volume, and mix. This solution contains 10.0 µg of copper per mL. Store in a polyethylene bottle.
Test preparation—
Transfer 5.0 g of Sodium Bicarbonate to a 100-mL plastic volumetric flask, and carefully add 4 mL of nitric acid. Sonicate for 30 minutes, dilute with water to volume, and mix.
Procedure—
To 10.0 mL of the Test preparation add 20 µL of Standard preparation, and mix. This Spiked test preparation contains 0.02 µg of added Cu per mL. Concomitantly determine the absorbances of the Test preparation and the Spiked test preparation at the copper emission line at 324.7 nm with a suitable atomic absorption spectrophotometer (see Spectrophotometry and Light-Scattering  851
851 ) equipped with a copper hollow-cathode lamp and a flameless electrically heated furnace, using Nitric acid diluent as the blank. Plot the absorbances of the Test preparation and the Spiked test preparation versus their contents of added Cu, in µg per mL, draw a line connecting the two points, and extrapolate the line until it intercepts the concentration axis. From the intercept determine the quantity, in µg, of Cu in each mL of the Test preparation. Calculate the quantity of Cu in the specimen tested by multiplying this value by 20: the limit is 1 µg per g.
) equipped with a copper hollow-cathode lamp and a flameless electrically heated furnace, using Nitric acid diluent as the blank. Plot the absorbances of the Test preparation and the Spiked test preparation versus their contents of added Cu, in µg per mL, draw a line connecting the two points, and extrapolate the line until it intercepts the concentration axis. From the intercept determine the quantity, in µg, of Cu in each mL of the Test preparation. Calculate the quantity of Cu in the specimen tested by multiplying this value by 20: the limit is 1 µg per g.
Iron  241
241 (where it is labeled as intended for use in hemodialysis)—
Place 2.0 g of Sodium Bicarbonate in a beaker, and neutralize with hydrochloric acid, noting the volume of acid consumed. Transfer this solution to a 25-mL volumetric flask with the aid of water (Test preparation). Prepare the Standard preparation by transferring 1.0 mL of Standard Iron Solution to a 25-mL volumetric flask and adding the same volume of hydrochloric acid as used to prepare the Test preparation. Prepare a Blank by adding the same volume of hydrochloric acid to a third 25-mL volumetric flask. To each of the flasks containing the Standard preparation, the Test preparation, and the Blank add 50 mg of ammonium peroxydisulfate crystals and 2 mL of Ammonium Thiocyanate Solution, dilute with water to volume, and mix. Concomitantly determine the absorbances of the solutions from the Standard preparation and the Test preparation at the wavelength of maximum absorbance at about 480 nm with a suitable spectrophotometer, using the solution from the Blank to set the instrument to zero. The absorbance of the solution from the Test preparation is not greater than that of the solution from the Standard preparation: not more than 5 µg per g is found.
(where it is labeled as intended for use in hemodialysis)—
Place 2.0 g of Sodium Bicarbonate in a beaker, and neutralize with hydrochloric acid, noting the volume of acid consumed. Transfer this solution to a 25-mL volumetric flask with the aid of water (Test preparation). Prepare the Standard preparation by transferring 1.0 mL of Standard Iron Solution to a 25-mL volumetric flask and adding the same volume of hydrochloric acid as used to prepare the Test preparation. Prepare a Blank by adding the same volume of hydrochloric acid to a third 25-mL volumetric flask. To each of the flasks containing the Standard preparation, the Test preparation, and the Blank add 50 mg of ammonium peroxydisulfate crystals and 2 mL of Ammonium Thiocyanate Solution, dilute with water to volume, and mix. Concomitantly determine the absorbances of the solutions from the Standard preparation and the Test preparation at the wavelength of maximum absorbance at about 480 nm with a suitable spectrophotometer, using the solution from the Blank to set the instrument to zero. The absorbance of the solution from the Test preparation is not greater than that of the solution from the Standard preparation: not more than 5 µg per g is found.
Heavy metals, Method I  231
231 —
Mix 4.0 g with 5 mL of water and 19 mL of 3 N hydrochloric acid, heat to boiling, and maintain that temperature for 1 minute. Add 1 drop of phenolphthalein TS, then add sufficient 6 N ammonium hydroxide, dropwise, to give the solution a faint pink color. Cool, and dilute with water to 25 mL: the limit is 5 µg per g.
—
Mix 4.0 g with 5 mL of water and 19 mL of 3 N hydrochloric acid, heat to boiling, and maintain that temperature for 1 minute. Add 1 drop of phenolphthalein TS, then add sufficient 6 N ammonium hydroxide, dropwise, to give the solution a faint pink color. Cool, and dilute with water to 25 mL: the limit is 5 µg per g.
Limit of ammonia—
Sodium hypochlorite solution—
Use a commercially available solution that contains 4.0% to 6.0% of sodium hypochlorite.
Oxidizing solution—
[note—Prepare on the day of use.] Prepare a mixture of alkaline sodium citrate TS and Sodium hypochlorite solution (4:1).
Diluted sodium nitroferricyanide solution—
Prepare a mixture of water and sodium nitroferricyanide TS (10:1).
Test solution—
Transfer 2.5 g of Sodium Bicarbonate to a 100-mL volumetric flask, dissolve in and dilute with water to volume, and mix.
Procedure—
[note—Carefully follow this order of addition stated below.] To 4.0 mL of the Test solution, add 0.4 mL of phenol TS, 0.4 mL of Diluted sodium nitroferricyanide solution, and 1.0 mL of Oxidizing solution. Dilute with water to 10 mL, mix, and allow to stand for 1 hour: no blue color develops.
Limit of organics—
(where it is labeled as intended for use in hemodialysis)—
Silver sulfate solution—
Dissolve 22 g of silver sulfate in 2000 mL of sulfuric acid.
Indicator solution—
Dissolve 1.485 g of 1,10-phenanthroline and 695 mg of ferrous sulfate in water to make 100 mL of solution.
Standard preparation—
Transfer 850.3 mg of potassium biphthalate, previously crushed lightly and dried at 120 for 2 hours, to a 1000-mL volumetric flask, dilute with water to volume, and mix. Transfer 6.0 mL of this solution to a 100-mL volumetric flask, dilute with water to volume, and mix. This solution contains the equivalent of 0.06 mg of organics equivalents per mL. Transfer 40.0 mL of this solution to a 500-mL reflux flask.
for 2 hours, to a 1000-mL volumetric flask, dilute with water to volume, and mix. Transfer 6.0 mL of this solution to a 100-mL volumetric flask, dilute with water to volume, and mix. This solution contains the equivalent of 0.06 mg of organics equivalents per mL. Transfer 40.0 mL of this solution to a 500-mL reflux flask.
Test preparation—
Transfer about 20 g of Sodium Bicarbonate, accurately weighed, to a 500-mL reflux flask. Add 20 mL of water, and swirl. Cautiously add 20 mL of sulfuric acid, and swirl. [Caution—Perform this operation under a hood.
]
Blank—
Add 40 mL of water to a 500-mL reflux flask.
Procedure—
Concomitantly treat the Standard preparation, the Test preparation, and the Blank as follows. Add 1 g of mercuric sulfate and about 5 glass beads. Cool the flask in an ice bath, and add 5 mL of Silver sulfate solution. While gently swirling the flask in the ice bath, add 25.0 mL of 0.025 N potassium dichromate VS and, slowly, 70 mL of Silver sulfate solution. Fit a cold water condenser on the reflux flask, and reflux for 2 hours. Allow the contents of the flask to cool for 10 minutes, and wash the condenser with 50 mL of water, collecting the washings in the flask. Add water to the flask to obtain a volume of about 350 mL. Add 3 drops of Indicator solution, and titrate, at room temperature, with 0.07 N ferrous ammonium sulfate VS until the solution changes from greenish blue to reddish brown. Calculate the amount, in mg, of organics equivalent in the Standard preparation taken by the formula:
8N(VB 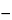 VS)
VS)
in which N is the normality of the ferrous ammonium sulfate VS; and VB and VS are the volumes, in mL, of 0.07 N ferrous ammonium sulfate VS consumed by the Blank and the Standard preparation, respectively. In a suitable system, between 2.328 and 2.424 mg is found. Calculate the amount, in mg, of organics equivalent in the portion of Sodium Bicarbonate taken by the formula:
8N(VB 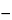 VU)
VU)
in which VU is the volume, in mL, of 0.07 N ferrous ammonium sulfate VS consumed by the Test preparation: the limit is 0.01%.
Assay—
Weigh accurately about 3 g of Sodium Bicarbonate, mix with 100 mL of water, add methyl red TS, and titrate with 1 N hydrochloric acid VS. Add the acid slowly, with constant stirring, until the solution becomes faintly pink. Heat the solution to boiling, cool, and continue the titration until the faint pink color no longer fades after boiling. Each mL of 1 N hydrochloric acid is equivalent to 84.01 mg of NaHCO3.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Elena Gonikberg, Ph.D.
Senior Scientist 1-301-816-8251 |
(MDGRE05) Monograph Development-Gastrointestinal Renal and Endocrine |
USP32–NF27 Page 3563
Pharmacopeial Forum: Volume No. 32(5) Page 1465
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.