Histamine Phosphate Injection
» Histamine Phosphate Injection is a sterile solution of Histamine Phosphate in Water for Injection. It contains not less than 90.0 percent and not more than 110.0 percent of the labeled amount of C5H9N3·2H3PO4.
Packaging and storage—
Preserve in single-dose or in multiple-dose containers, preferably of Type I glass, protected from light.
Identification—
A:
Evaporate a volume of Injection, equivalent to about 2 mg of histamine phosphate, on a steam bath to dryness, dissolve the residue in 0.5 mL of water, and add 0.5 mL of 1 N sodium hydroxide. Add 2 drops of sodium nitrite solution (1 in 10), and add 1 mL of a solution prepared by mixing 50 mg of sulfanilic acid with 10 mL of water containing 2 drops of hydrochloric acid: an orange-red color is produced.
B:
To 1 mL of Injection, equivalent to not less than 1 mg of histamine phosphate (concentrate a larger volume by evaporation, if necessary), add ammonium molybdate TS dropwise: a yellow precipitate, which is soluble in ammonia TS, is formed.
Bacterial endotoxins  85
85 —
It contains not more than 125.0 USP Endotoxin Units per mg of histamine phosphate.
—
It contains not more than 125.0 USP Endotoxin Units per mg of histamine phosphate.
pH  791
791 :
between 3.0 and 6.0.
:
between 3.0 and 6.0.
Other requirements—
It meets the requirements under Injections  1
1 .
.
Assay—
Standard preparation—
Dissolve an accurately weighed quantity of USP Histamine Dihydrochloride RS in water, and quantitatively dilute with water to obtain a solution having a known concentration of 20 µg per mL, equivalent to 33.4 µg of histamine phosphate.
Assay preparation—
Dilute an accurately measured volume of Injection, equivalent to about 1.65 mg of histamine phosphate, with water in a 50-mL volumetric flask to volume, and mix.
If phenol is present
, prepare the Assay preparation as follows. Dilute an accurately measured volume of Injection, equivalent to about 1.65 mg of histamine phosphate, with water to about 25 mL. Heat the solution on a steam bath until the odor of phenol is no longer perceptible, adding water as required to maintain a volume of about 15 mL. Transfer to a 50-mL volumetric flask, cool, dilute with water to volume, and mix.
Procedure—
Pipet 5 mL each of the Standard preparation and the Assay preparation into separate, 10-mL volumetric flasks, to each add 1 mL of sodium borate solution (1 in 100), followed by 1 mL of a freshly prepared solution of 50 mg of 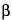 -naphthoquinone-4-sodium sulfonate in 10 mL of water. Place the flasks in boiling water for 10 minutes, then immerse them for 5 minutes in water maintained between 5
-naphthoquinone-4-sodium sulfonate in 10 mL of water. Place the flasks in boiling water for 10 minutes, then immerse them for 5 minutes in water maintained between 5 and 10
and 10 . To each flask, add 1 mL of acid-formaldehyde (made by adding 0.5 mL of formaldehyde TS to a mixture of 45 mL of 1 N hydrochloric acid and 10 mL of glacial acetic acid and diluting with water to 80 mL), mix, add 1 mL of 0.1 N sodium thiosulfate, then dilute with water to volume, and mix. Concomitantly and immediately determine the absorbances of both solutions at the wavelength of maximum absorbance at about 460 nm, with a suitable spectrophotometer, against a reagent blank. Calculate the quantity, in mg, of C5H9N3·2H3PO4 in each mL of the Injection taken by the formula:
. To each flask, add 1 mL of acid-formaldehyde (made by adding 0.5 mL of formaldehyde TS to a mixture of 45 mL of 1 N hydrochloric acid and 10 mL of glacial acetic acid and diluting with water to 80 mL), mix, add 1 mL of 0.1 N sodium thiosulfate, then dilute with water to volume, and mix. Concomitantly and immediately determine the absorbances of both solutions at the wavelength of maximum absorbance at about 460 nm, with a suitable spectrophotometer, against a reagent blank. Calculate the quantity, in mg, of C5H9N3·2H3PO4 in each mL of the Injection taken by the formula:
C(0.0835 / V)(AU / AS)
in which C is the concentration, in µg per mL, of USP Histamine Dihydrochloride RS in the Standard preparation; V is the volume, in mL, of Injection taken; and AU and AS are the absorbances of the solutions from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Elena Gonikberg, Ph.D.
Senior Scientist 1-301-816-8251 |
(MDGRE05) Monograph Development-Gastrointestinal Renal and Endocrine |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance |
USP32–NF27 Page 2558