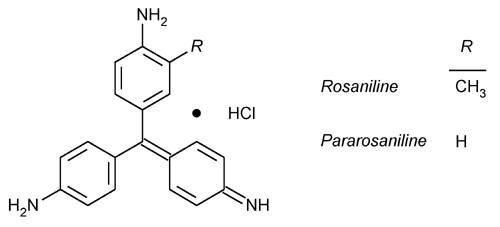
Basic Fuchsin
Benzenamine, 4-[(4-aminophenyl)(4-imino-2,5-cyclohexadien-1-ylidene)methyl-2-methyl]-, monohydrochloride.
C.I. Basic Violet 14 monohydrochloride
» Basic Fuchsin is a mixture of rosaniline and pararosaniline hydrochlorides. It contains the equivalent of not less than 88.0 percent of rosaniline hydrochloride (C20H19N3·HCl), calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers.
Identification—
A:
To 5 mL of a solution (1 in 1000) add a few drops of hydrochloric acid: a yellow color is produced (distinction from acid fuchsin).
B:
To 5 mL of a solution (1 in 500) add a few drops of tannic acid TS: a red precipitate is formed.
C:
To 10 mL of a solution (1 in 500) add 10 mL of ammonia TS and 500 mg of zinc dust, and agitate the mixture: the solution becomes decolorized. Place a few drops of the decolorized solution on filter paper, and nearby on the same paper place a few drops of 3 N hydrochloric acid: a red color develops at the zone of contact.
Loss on drying  731
731 —
Dry it at 105
—
Dry it at 105 to constant weight: it loses not more than 5.0% of its weight.
to constant weight: it loses not more than 5.0% of its weight.
Residue on ignition  281
281 —
Ignite 1 g with 0.5 mL of sulfuric acid: the weight of the residue is not more than 0.3%.
—
Ignite 1 g with 0.5 mL of sulfuric acid: the weight of the residue is not more than 0.3%.
Alcohol-insoluble substances—
Boil 1 g, accurately weighed, with 50 mL of alcohol under a reflux condenser for 15 minutes, filter through a tared filtering crucible, wash the residue on the filter with hot alcohol until the washings cease to be colored violet, and dry the crucible at 105 for 1 hour: the amount of insoluble residue is not more than 1.0%.
for 1 hour: the amount of insoluble residue is not more than 1.0%.
Arsenic, Method II  211
211 :
8 ppm.
:
8 ppm.
Lead  251
251 :
Place 1 g in a small Kjeldahl flask, add 5 mL of sulfuric acid, and insert a small funnel into the flask. Gently rotate the flask until the sulfuric acid has completely wetted the Basic Fuchsin, then heat with a small flame until carbonization is complete. Allow to cool, and add, in small quantities, 5 mL of nitric acid. Again heat gently until fumes of sulfur trioxide are evolved. Allow to cool, add another 5 mL of nitric acid, and heat to the evolution of sulfur trioxide. Allow to cool, add about 25 mL of water, and boil for a few minutes. Cool, neutralize with stronger ammonia water, using litmus paper as the indicator, and add 5 mL of nitric acid. Transfer the solution to a 100-mL volumetric flask, dilute to volume, and mix. A 20-mL portion of this solution contains not more than 30 ppm of lead.
:
Place 1 g in a small Kjeldahl flask, add 5 mL of sulfuric acid, and insert a small funnel into the flask. Gently rotate the flask until the sulfuric acid has completely wetted the Basic Fuchsin, then heat with a small flame until carbonization is complete. Allow to cool, and add, in small quantities, 5 mL of nitric acid. Again heat gently until fumes of sulfur trioxide are evolved. Allow to cool, add another 5 mL of nitric acid, and heat to the evolution of sulfur trioxide. Allow to cool, add about 25 mL of water, and boil for a few minutes. Cool, neutralize with stronger ammonia water, using litmus paper as the indicator, and add 5 mL of nitric acid. Transfer the solution to a 100-mL volumetric flask, dilute to volume, and mix. A 20-mL portion of this solution contains not more than 30 ppm of lead.
Assay—
Dissolve about 100 mg of Basic Fuchsin, accurately weighed, in 175 mL of water in a 500-mL closed system titration vessel fitted with a gas inlet tube, a gas outlet tube, an upright reflux condenser, and a buret. Add about 25 mL of sodium tartrate solution (30 in 100) and a polytef-coated magnetic stirring bar, and heat to boiling. Flush this titration vessel for 15 minutes with nitrogen that has been passed through two successive gas washing bottles each containing 500 mL of a mixture of water, titanium trichloride solution (20 in 100), and hydrochloric acid (400:40:40) to which about 10 mg of safranin O has been added. Continue the heating and nitrogen flow, and while stirring titrate with 0.05 N titanium trichloride VS to a yellow endpoint. Each mL of 0.05 N titanium trichloride is equivalent to 3.379 mg of C20H19N3·HCl.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Elena Gonikberg, Ph.D.
Senior Scientist 1-301-816-8251 |
(MDGRE05) Monograph Development-Gastrointestinal Renal and Endocrine |
USP32–NF27 Page 2457