Lipid Injectable Emulsion
» Lipid Injectable Emulsion used in total parenteral nutrition is a sterile 10 (0.10 g per mL), 20 (0.20 g per mL), or 30 (0.30 g per mL) percent w/v emulsion in a vehicle. The vehicle contains 2.25 percent to 2.5 percent w/v Glycerin and 0.74 percent to 1.8 percent w/v parenteral Egg Phospholipids in Water for Injection. The principal oil used is Soybean Oil, which provides an ample supply of the essential fatty acids: linoleic acid and linolenic acid. Other oils, such as Safflower Oil, Medium-Chain Triglycerides, Olive Oil, Fish Oil, or other suitable oils, can be mixed with Soybean Oil. Hence, Soybean Oil can be the only oil or be part of a mixture of these other oils. It contains not less than 90.0 percent and not more than 110.0 percent of the labeled amount of the oils. It contains no antimicrobial agents. The final products are terminally sterilized.
Packaging and storage—
Preserve in an appropriate container (see Injections  1
1 ). Use elastomeric closures that are compatible with both the oil and water phases of the Emulsion. Store at a temperature not below 4
). Use elastomeric closures that are compatible with both the oil and water phases of the Emulsion. Store at a temperature not below 4 (protect from freezing) or above 30
(protect from freezing) or above 30 (protect from excessive heat).
(protect from excessive heat).
Labeling—
The label states the identity and the quantities of the specific oils in the Emulsion. The label states the total osmolar concentration (or osmolarity) in mOsm per L. The labeling provides the following information: do not use if there is evidence of excessive creaming or aggregation, if excessive free oil droplets are visible, or if there are other indications of compromised integrity, such as microbial growth, present in the product.
Fatty acid composition—
Transfer a volume of the Emulsion, equivalent to about 200 mg of lipids, to a stoppered extraction vessel, add 10 mL of ether, and mix. Add 5 g of anhydrous sodium sulfate, mix, and allow the mixture to stand until separation of the layers is complete. Wet the packing of a chromatographic silica cartridge with a few mL of ether, transfer about 5 mL of the ether layer from the extraction vessel to the column reservoir, and elute at a rate of between 5 and 10 drops per minute into a suitable vessel. Evaporate the ether from the eluant, and dissolve the residue in 5.0 mL of toluene. Transfer 1.0 mL of the toluene solution to a reaction vial, and add 0.4 mL of (m-trifluoromethylphenyl) trimethylammonium hydroxide in methanol. Cover, mix, and allow to stand for 30 minutes. Inject about 1 µL of this solution into a gas chromatograph equipped with a 0.53-mm × 50-m wide-bore, fused-silica capillary column coated with a 2.0-µm thickness of liquid phase G16 and maintained at a temperature of 200 . Helium is used as the carrier gas at a flow rate of about 10 mL per minute. Measure the main peak areas of the methyl esters of the fatty acids. The relative peak areas expressed as a percentage of the main peaks are in the known ranges for the oil (e.g., Soybean Oil, USP; Safflower Oil, USP) as specified on the label. For oil mixtures, analysis of each oil should be performed to identify known peaks prior to emulsification as specified on the label.
. Helium is used as the carrier gas at a flow rate of about 10 mL per minute. Measure the main peak areas of the methyl esters of the fatty acids. The relative peak areas expressed as a percentage of the main peaks are in the known ranges for the oil (e.g., Soybean Oil, USP; Safflower Oil, USP) as specified on the label. For oil mixtures, analysis of each oil should be performed to identify known peaks prior to emulsification as specified on the label.
Bacterial endotoxins  85
85 —
It contains not more than 0.5 USP Endotoxin Unit per mL.
—
It contains not more than 0.5 USP Endotoxin Unit per mL.
pH  791
791 :
between 6.0 and 9.0.
:
between 6.0 and 9.0.
Globule size limits—
Lipid Injectable Emulsion meets the requirements of the limits specified in both Method I and Method II as directed under Globule Size Distribution in Lipid Injectable Emulsions  729
729 .
.
Limit of oil droplet mean diameters
(See Method I—Light Scattering Method under Globule Size Distribution in Lipid Injectable Emulsions  729
729 )—Using the method of light scattering, determine the mean droplet diameter (MDD): the sample meets the requirements. The intensity-weighted mean droplet diameter (MDD) for Lipid Injectable Emulsion must be
)—Using the method of light scattering, determine the mean droplet diameter (MDD): the sample meets the requirements. The intensity-weighted mean droplet diameter (MDD) for Lipid Injectable Emulsion must be 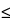 500 nm, or 0.5 µm, irrespective of the concentration of the dispersed lipid phase.
500 nm, or 0.5 µm, irrespective of the concentration of the dispersed lipid phase.
Limit of large globule volume-diameter
(See Method II—Light Obscuration or Extinction Method under Globule Size Distribution in Lipid Injectable Emulsions  729
729 )—Using the method of light obscuration, determine the size distribution of globules in the large-diameter tail of the dispersion (detection threshold
)—Using the method of light obscuration, determine the size distribution of globules in the large-diameter tail of the dispersion (detection threshold 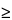 2.0 µm). Calculate the volume-weighted mass of lipid in the form of globules with diameters in excess of 5.0 µm per 100 mL of Emulsion. This mass does not exceed 0.05% of the dispersed phase that is
2.0 µm). Calculate the volume-weighted mass of lipid in the form of globules with diameters in excess of 5.0 µm per 100 mL of Emulsion. This mass does not exceed 0.05% of the dispersed phase that is 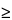 5.0 µm from the nominal lipid concentration stated on the label.
5.0 µm from the nominal lipid concentration stated on the label.
Limit of free fatty acid—
Solvent—
Prepare a mixture of heptane, isopropanol, and water (400:400:200) in a separatory funnel. Allow the phases to separate, and discard the lower phase. Filter the upper phase (heptane solution) through 40 g of anhydrous sodium sulfate. Store in a tightly capped glass container, and use within 1 week.
Chromatographic column—
Prepare a slurry of heptane and chromatographic silica gel having an average pore size of 6 nm, and activate at a temperature of 110 for not less than 1 hour prior to use. Transfer the slurry to a 2.3-cm chromatographic tube (see Column Chromatography under Chromatography
for not less than 1 hour prior to use. Transfer the slurry to a 2.3-cm chromatographic tube (see Column Chromatography under Chromatography  621
621 ), and pack to a bed height of between 5 cm and 6 cm. Wash the column with about 40 mL of heptane, and drain the heptane through the column to a level of about 0.5 cm above the silica gel bed.
), and pack to a bed height of between 5 cm and 6 cm. Wash the column with about 40 mL of heptane, and drain the heptane through the column to a level of about 0.5 cm above the silica gel bed.
Procedure
—Transfer 20.0 mL of the Emulsion to a flask, freeze, and lyophilize. Dissolve the residue in 30 mL of Solvent, and transfer the solution to the column. Rinse the flask with three 30-mL portions of Solvent, and transfer the washings to the column, allowing each rinsing to drain to the top of the column bed before applying the next rinse. Collect a total of 120 mL of effluent. Add 10 drops of phenolphthalein TS to the effluent, bubble nitrogen through the solution, and titrate with 0.02 N alcoholic potassium hydroxide VS until the solution remains pale pink after mixing for 10 seconds. Titrate a blank using 120 mL of Solvent. Calculate the quantity, in mEq, of free fatty acids in the portion of Emulsion taken by the formula:
(VU – VB)N / 20C
in which VU is the volume, in mL, of 0.02 N alcoholic potassium hydroxide consumed by the eluant; VB is the volume, in mL, of 0.02 N alcoholic potassium hydroxide consumed by the blank; N is the normality of the 0.02 N alcoholic potassium hydroxide; and C is the labeled concentration, in g per mL, of the oils in the Emulsion: not more than 0.07 mEq of free fatty acids per g of Emulsion is found.
Other requirements—
It meets the requirements under Injections  1
1 .
.
Assay—
Mobile phase
—Prepare a filtered and degassed mixture of isopropanol, ethyl acetate, and glacial acetic acid (179:20:1).
Standard preparation
—Dissolve an accurately weighed portion of Soybean Oil (or other relevant oils used in the Emulsion) in Mobile phase to obtain a solution having a known concentration of about 8 mg per mL.
Assay preparation
—Transfer an accurately measured portion of Emulsion, equivalent to about 800 mg of oil, to a 100-mL volumetric flask with the aid of additional portions of Mobile phase. Dilute with Mobile phase to volume, and mix to obtain a solution containing about 8 mg of oil per mL.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a refractive index detector and a 4.1-mm × 25-cm column that contains packing L21. The flow rate is about 1 mL per minute, adjusted so that the peak due to oil elutes at about 6.5 minutes. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the capacity factor, k¢, is not less than 1.0; the tailing factor for the oil peak is not more than 2.5; and the relative standard deviation for replicate injections is not more than 2.0%.
)—
The liquid chromatograph is equipped with a refractive index detector and a 4.1-mm × 25-cm column that contains packing L21. The flow rate is about 1 mL per minute, adjusted so that the peak due to oil elutes at about 6.5 minutes. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the capacity factor, k¢, is not less than 1.0; the tailing factor for the oil peak is not more than 2.5; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure
—Separately inject equal volumes (about 50 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the peak responses. Calculate the quantity, in mg, of oil in the portion of Emulsion taken by the formula:
100C(rU / rS)
in which C is the concentration, in mg per mL, of Soybean Oil or other relevant oils used in the Emulsion in the Standard preparation; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Desmond G. Hunt, Ph.D.
Scientist 1-301-816-8341 |
(PPI05) Parenteral Products-Industrial 05 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance |
USP32–NF27 Page 2793
Pharmacopeial Forum: Volume No. 34(3) Page 621