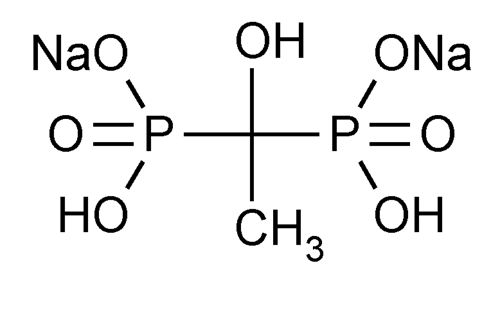
Etidronate Disodium
Phosphonic acid, (1-hydroxyethylidene)bis-, disodium salt.
Disodium dihydrogen (1-hydroxyethylidene)diphosphonate
» Etidronate Disodium contains not less than 97.0 percent and not more than 101.0 percent of C2H6Na2O7P2, calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
USP Reference standards  11
11 —
—
USP Etidronate Disodium RS.
USP Etidronate Disodium Related Compound A RS.
USP Etidronic Acid Monohydrate RS.
USP Etidronate Disodium RS.
USP Etidronate Disodium Related Compound A RS.
USP Etidronic Acid Monohydrate RS.
Identification—
A:
Infrared Absorption  197
197 —The spectra of trifluorovinyl chloride polymer and mineral oil dispersions of it, separately prepared from a test specimen recrystallized from water and dried at 105
—The spectra of trifluorovinyl chloride polymer and mineral oil dispersions of it, separately prepared from a test specimen recrystallized from water and dried at 105 for 1 hour, exhibit maxima in the regions of 4000 to 1350 cm-1 and 1350 to 450 cm-1, respectively, only at the same wavelengths as those of similar preparations of USP Etidronate Disodium RS.
for 1 hour, exhibit maxima in the regions of 4000 to 1350 cm-1 and 1350 to 450 cm-1, respectively, only at the same wavelengths as those of similar preparations of USP Etidronate Disodium RS.
B:
A solution (1 in 100) meets the requirements of the flame test for Sodium  191
191 .
.
pH  791
791 :
between 4.2 and 5.2, in a solution (1 in 100).
:
between 4.2 and 5.2, in a solution (1 in 100).
Water, Method Ic  921
921 :
not more than 5.0%, determined on a preparation containing 100 mg of finely powdered etidronate disodium in 10 mL of a mixture of acetic acid and formamide (1:1).
:
not more than 5.0%, determined on a preparation containing 100 mg of finely powdered etidronate disodium in 10 mL of a mixture of acetic acid and formamide (1:1).
Limit of phosphite—
Solution A—
Prepare an aqueous solution containing 0.65 mg per mL of anhydrous sodium carbonate and 0.40 mg per mL of sodium bicarbonate.
Solution B—
Prepare an aqueous solution containing 4.68 mg per mL of anhydrous sodium carbonate and 2.89 mg per mL of sodium bicarbonate.
Mobile phase—
Use variable mixtures of Solution A and Solution B as directed for Chromatographic system. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard solution—
Dissolve suitable quantities of USP Etidronate Disodium Related Compound A RS and dibasic sodium phosphate in Solution A to obtain a solution having a known concentration of 0.016 mg of dibasic sodium phosphite on the anhydrous basis and 0.015 mg of dibasic sodium phosphate in each mL. [note—Etidronate disodium related compound A is dibasic sodium phosphite pentahydrate.]
Suppressor regenerant solution—
Use 12.5 mM sulfuric acid. [note—This solution is needed only if the chemical suppression option is used.]
Test solution—
Transfer approximately 50 mg of Etidronate Disodium, accurately weighed, to a suitable flask. Dissolve in 10.0 mL of Solution A.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a conductivity detector, a 4-mm × 25-cm column and a 4-mm × 50-mm guard column both containing packing L61, and either a 4-mm anion self-regenerating suppressor or a suitable chemical suppressor. The flow rate is about 1.0 mL per minute for the Mobile phase. When a chemical suppressor is used, the flow rate is 3 to 5 mL per minute for the Suppressor regenerant solution. The chromatograph is programmed as follows.
)—
The liquid chromatograph is equipped with a conductivity detector, a 4-mm × 25-cm column and a 4-mm × 50-mm guard column both containing packing L61, and either a 4-mm anion self-regenerating suppressor or a suitable chemical suppressor. The flow rate is about 1.0 mL per minute for the Mobile phase. When a chemical suppressor is used, the flow rate is 3 to 5 mL per minute for the Suppressor regenerant solution. The chromatograph is programmed as follows.
Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the elution order is phosphite, followed by phosphate; the resolution, R, between phosphite and phosphate is not less than 2.5; and the relative standard deviation for replicate injections is not more than 10% for each peak.
| Time (minutes) |
Solution A
(%) |
Solution B
(%) |
|
| 0–6.0 | 100 | 0 | isocratic |
| 6.0–6.1 | 100®0 | 0®100 | linear gradient |
| 6.1–8.0 | 0 | 100 | isocratic |
| 8.0–8.1 | 0®100 | 100®0 | linear gradient |
| 8.1–15 | 100 | 0 | isocratic |
Procedure—
Separately inject equal volumes (about 20 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the responses for the phosphite peaks. Calculate the percentage of phosphite, determined as monobasic sodium phosphite, in the portion of Etidronate Disodium taken by the formula:
1000(103.98/125.96)(C/W)(rU / rS)
in which 103.98 and 125.96 are the molecular weights of monobasic sodium phosphite and dibasic sodium phosphite, respectively; C is the concentration, in mg per mL, of USP Etidronate Disodium Related Compound A RS on the anhydrous basis in the Standard solution; W is the weight, in mg, of Etidronate Disodium taken to prepare the Test solution; and rU and rS are the phosphite peak responses obtained from the Test solution and the Standard solution, respectively: not more than 1.0% of phosphite, determined as monobasic sodium phosphite, is found.
Heavy metals, Method II  231
231 —
Use 0.5 g of Etidronate Disodium for the Test Preparation and 2.5 mL of Standard Lead Solution for the Standard Preparation. Transfer the Test Preparation and the Standard Preparation to separate quartz crucibles, add 0.5 g of magnesium oxide to each crucible, and mix. Evaporate the Standard Preparation to dryness at 110
—
Use 0.5 g of Etidronate Disodium for the Test Preparation and 2.5 mL of Standard Lead Solution for the Standard Preparation. Transfer the Test Preparation and the Standard Preparation to separate quartz crucibles, add 0.5 g of magnesium oxide to each crucible, and mix. Evaporate the Standard Preparation to dryness at 110 for 1 hour, and ignite each crucible over a flame to a light gray color. Ignite at 800
for 1 hour, and ignite each crucible over a flame to a light gray color. Ignite at 800 for 1 hour, cool, and dissolve the residues by the dropwise addition of hydrochloric acid, and add 3 mL of water. Adjust with ammonia TS to a pH of 8.5, then adjust with acetic acid to a pH of 4. Make a final pH adjustment to 3.4 ± 0.05, using dilute hydrochloric acid. Filter into 50-mL color comparison tubes, and dilute with water to 40 mL. The limit is 0.005%.
for 1 hour, cool, and dissolve the residues by the dropwise addition of hydrochloric acid, and add 3 mL of water. Adjust with ammonia TS to a pH of 8.5, then adjust with acetic acid to a pH of 4. Make a final pH adjustment to 3.4 ± 0.05, using dilute hydrochloric acid. Filter into 50-mL color comparison tubes, and dilute with water to 40 mL. The limit is 0.005%.
Assay—
Mobile phase—
Prepare a 35 mM to 40 mM ammonium nitrate solution in water, and adjust with dilute ammonium hydroxide to a pH of 7.0.
Standard preparation—
Dissolve an accurately weighed quantity of USP Etidronic Acid Monohydrate RS in a mixture of 1 mL of 1 N sodium hydroxide solution and 150 mL of Mobile phase, to obtain a solution having a known concentration between 0.73 and 0.75 mg of etidronic acid monohydrate per mL.
Assay preparation—
Transfer between 42.0 and 43.0 mg of Etidronate Disodium, accurately weighed, to a 50-mL volumetric flask, and dissolve in and dilute with Mobile phase to volume.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a refractive index detector and a 4.6- × 150-mm column that contains packing L23. The column and the detector temperatures are maintained at 32
)—
The liquid chromatograph is equipped with a refractive index detector and a 4.6- × 150-mm column that contains packing L23. The column and the detector temperatures are maintained at 32 . The flow rate is about 1.5 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 1.5%.
. The flow rate is about 1.5 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 1.5%.
Procedure—
Separately inject equal volumes (about 20 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the percentage of C2H6Na2O7P2 in the portion of Etidronate Disodium taken by the formula:
100(250.00/224.05)(CS / CU)(rU / rS)
in which 250.00 and 224.05 are the molecular weights of etidronate disodium and etidronic acid monohydrate, respectively; CS is the concentration, in mg per mL, of USP Etidronic Acid Monohydrate RS in the Standard preparation; CU is the concentration, in mg per mL, of Etidronate Disodium in the Assay preparation; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Elena Gonikberg, Ph.D.
Senior Scientist 1-301-816-8251 |
(MDGRE05) Monograph Development-Gastrointestinal Renal and Endocrine |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2333
Pharmacopeial Forum: Volume No. 33(4) Page 648
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.