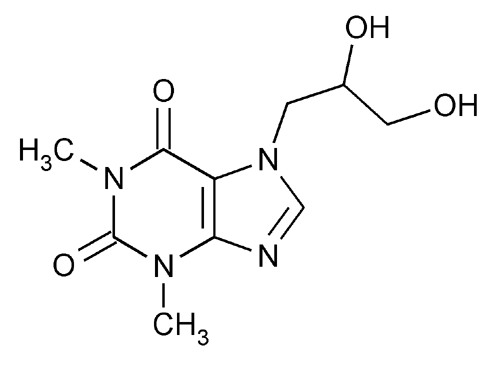
Dyphylline
1H-Purine-2,6-dione, 7-(2,3-dihydroxypropyl)-3,7-dihydro-1,3-dimethyl-, (±)-.
(±)-7-(2,3-Dihydroxypropyl)theophylline
» Dyphylline contains not less than 98.0 percent and not more than 102.0 percent of C10H14N4O4, calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
Identification—
Solution:
10 µg per mL.
Medium:
water.
pH  791
791 :
between 5.0 and 7.5, in a solution (1 in 100).
:
between 5.0 and 7.5, in a solution (1 in 100).
Loss on drying  731
731 —
Dry about 1 g, accurately weighed, at 105
—
Dry about 1 g, accurately weighed, at 105 for 3 hours: it loses not more than 0.5% of its weight.
for 3 hours: it loses not more than 0.5% of its weight.
Residue on ignition  281
281 :
not more than 0.15%.
:
not more than 0.15%.
Chloride  221
221 :
A 1.0-g portion shows no more chloride than corresponds to 0.50 mL of 0.020 N hydrochloric acid (0.035%).
:
A 1.0-g portion shows no more chloride than corresponds to 0.50 mL of 0.020 N hydrochloric acid (0.035%).
Sulfate  221
221 :
A 1.0-g portion shows no more sulfate than corresponds to 0.10 mL of 0.020 N sulfuric acid (0.010%).
:
A 1.0-g portion shows no more sulfate than corresponds to 0.10 mL of 0.020 N sulfuric acid (0.010%).
Heavy metals, Method I  231
231 :
0.002%.
:
0.002%.
Limit of theophylline—
Dissolve 2.0 g in 10 mL of hot water. Cool, add 10 drops of bromothymol blue TS and 5.0 mL of silver nitrate TS, and mix. Not more than 0.50 mL of 0.10 N sodium hydroxide is required to change the color of the solution to blue (0.5%).
Related compounds—
Dissolve about 200 mg in a mixture of 30 volumes of methanol and 20 volumes of water in a 10-mL volumetric flask, dilute with the same mixture to volume, and mix (Test solution). Dilute 1 mL of the Test solution with methanol to 100 mL (Diluted test solution). Separately apply 10 µL of each solution to a suitable thin-layer chromatographic plate (see Chromatography  621
621 ) coated with a 0.25-mm layer of binder-free chromatographic silica gel mixture. Develop the plate in a suitable chamber containing a mixture of chloroform, dehydrated alcohol, and ammonium hydroxide (90:10:1) until the solvent front has moved about 15 cm. Remove the plate, air-dry at room temperature, and examine under short-wavelength UV light: no spot in the chromatogram of the Test solution other than the principal spot is larger or more intense than the principal spot from the Diluted test solution (1.0%).
) coated with a 0.25-mm layer of binder-free chromatographic silica gel mixture. Develop the plate in a suitable chamber containing a mixture of chloroform, dehydrated alcohol, and ammonium hydroxide (90:10:1) until the solvent front has moved about 15 cm. Remove the plate, air-dry at room temperature, and examine under short-wavelength UV light: no spot in the chromatogram of the Test solution other than the principal spot is larger or more intense than the principal spot from the Diluted test solution (1.0%).
Assay—
Dissolve about 200 mg of Dyphylline, accurately weighed, in 2 mL of formic acid, and carefully add, with stirring, 50 mL of acetic anhydride. Add 0.2 mL of Sudan IV TS, and titrate with 0.1 N perchloric acid VS to a deep violet endpoint. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 25.42 mg of C10H14N4O4.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Daniel K. Bempong, Ph.D.
Senior Scientist 1-301-816-8143 |
(MDPS05) Monograph Development-Pulmonary and Steroids |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2223
Pharmacopeial Forum: Volume No. 29(5) Page 1473
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.