Caprylocaproyl Polyoxylglycerides
(Title for this new monograph—to become official April 1, 2010)
(Prior to April 1, 2010, it is expected that the current practice of labeling the article of commerce with the name Caprylocaproyl Macrogolglycerides will be continued.)
» Caprylocaproyl Polyoxylglycerides are mixtures of monoesters, diesters, and triesters of glycerol and monoesters and diesters of polyethylene glycols with a mean relative molecular weight between 200 and 400. They are produced by partial alcoholysis of medium-chain triglycerides with polyethylene glycol, by esterification of glycerol and polyethylene glycol with caprylic acid and capric acid, or as a mixture of glycerol esters and ethylene oxide condensate with caprylic acid and capric acid. The average molecular weight is not less than 90.0 percent and not more than 110.0 percent of the labeled nominal value.
Packaging and storage—
Preserve in tight containers, protected from light and moisture. Store at controlled room temperature.
Labeling—
Label it to indicate the average nominal molecular weight of esters as part of the official title. The label also indicates the nominal hydroxyl value and saponification value.
Identification—
B:
Thin-Layer Chromatographic Identification Test  201
201 —
—
Test solution:
0.05 g per mL, prepared by dissolving 1.0 g of Caprylocaproyl Polyoxylglycerides in methylene chloride, and diluting with methylene chloride to 20 mL.
Developing solvent system:
a mixture of ether and solvent hexane (7:3).
Procedure—
Proceed as directed in the chapter (except for Caprylocaproyl Polyglycerides apply 50 µL of the Test solution and 50 µL of the Standard solution). Then spray the plate with reagent solution, prepared by dissolving 0.01 g of rhodamine 6G in 100 mL of isopropyl alcohol, and examine the plate under UV light at 365 nm: the RF values of the principle spots obtained from the Test solution correspond to those obtained from the Standard solution.
Acid value  401
401 :
not more than 2.0, determined on a 2.0-g specimen.
:
not more than 2.0, determined on a 2.0-g specimen.
Hydroxyl value  401
401 —
The hydroxyl value, between 170 and 205, does not differ by more than 20 units from the nominal value, when determined on a 1.0-g specimen, accurately weighed.
—
The hydroxyl value, between 170 and 205, does not differ by more than 20 units from the nominal value, when determined on a 1.0-g specimen, accurately weighed.
Iodine value  401
401 :
not more than 2.0.
:
not more than 2.0.
Peroxide value  401
401 :
not more than 6.0, determined on a 2.0-g specimen.
:
not more than 6.0, determined on a 2.0-g specimen.
Saponification value  401
401 —
The saponification value, between 85 and 105, does not differ by more than 10 units from the nominal value, determined on a 2.0-g specimen.
—
The saponification value, between 85 and 105, does not differ by more than 10 units from the nominal value, determined on a 2.0-g specimen.
Fatty acid composition  401
401 :
between 50% and 80% of caprylic acid is found; not more than 2.0% of caproic acid is found; between 20% and 50% of capric acid is found; not more than 3.0% of lauric acid is found; and not more than 1.0% of myristic acid is found.
:
between 50% and 80% of caprylic acid is found; not more than 2.0% of caproic acid is found; between 20% and 50% of capric acid is found; not more than 3.0% of lauric acid is found; and not more than 1.0% of myristic acid is found.
Water, Method I  921
921 :
not more than 1.0%, determined on a 1.0-g specimen.
:
not more than 1.0%, determined on a 1.0-g specimen.
Total ash  561
561 :
not more than 0.1%, determined on a 1.0-g specimen.
:
not more than 0.1%, determined on a 1.0-g specimen.
Heavy metals, Method II  231
231 :
0.001%.
:
0.001%.
Limit of free ethylene oxide and dioxane—
Caution—Ethylene oxide is toxic and flammable. Prepare all solutions in a well-ventilated hood. The operator must protect hands and face by wearing polyethylene protective gloves and an appropriate face mask. Store all solutions in hermetic containers, and refrigerate at a temperature between 4 and 8
and 8 .
.
note—Perform all determinations three times.
Ethylene oxide stock solution—
Into a dry, clean test tube, cooled in a mixture of 1 part of sodium chloride and 3 parts of crushed ice, introduce a slow current of ethylene oxide gas, allowing condensation onto the inner wall of the test tube. Using a glass syringe, previously cooled to 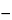 10
10 , transfer about 300 µL of liquid ethylene oxide, equivalent to about 0.25 g, to 50 mL of polyethylene glycol 200. Determine the absorbed quantity of ethylene oxide by weighing before and after absorption. Dilute with polyethylene glycol 200 to 100.0 mL, and mix. This is the Ethylene oxide stock solution.
, transfer about 300 µL of liquid ethylene oxide, equivalent to about 0.25 g, to 50 mL of polyethylene glycol 200. Determine the absorbed quantity of ethylene oxide by weighing before and after absorption. Dilute with polyethylene glycol 200 to 100.0 mL, and mix. This is the Ethylene oxide stock solution.
Transfer 10.0 mL of magnesium chloride solution, prepared by adding 5 g of magnesium chloride to 10 mL of alcohol, to a volumetric flask. Add 20.0 mL of 0.1 M alcoholic hydrochloric acid VS. Insert the stopper, shake to obtain a saturated solution, and allow to equilibrate overnight. Transfer 5.00 mL of Ethylene oxide stock solution, accurately measured, to the flask, and allow to stand for 30 minutes. Titrate with 0.1 M alcoholic potassium hydroxide VS, determining the endpoint potentiometrically. Perform a blank titration, using the same quantity of polyethylene glycol 200 instead of Ethylene oxide stock solution, and note the difference in volumes required. Each mL of the difference in volumes of 0.1 M alcoholic potassium hydroxide VS consumed is equivalent to 4.404 mg of ethylene oxide. Calculate the concentration of ethylene oxide, in mg per g, in the Ethylene oxide stock solution.
Ethylene oxide solution—
Quantitatively dilute a volume of Ethylene oxide stock solution, accurately measured, with polyethylene glycol 200 to obtain a solution containing about 50 µg of ethylene oxide per g. Dilute 1.0 mL of this solution with water to 5.0 mL to obtain a solution having a known concentration of about 10 µg of ethylene oxide per mL. [note—Prepare immediately before use.]
Dioxane stock solution—
Dissolve about 1.00 g of dioxane, accurately weighed, in water, and dilute quantitatively, and stepwise if necessary, with water to obtain a solution having a known concentration of about 1.0 mg per mL.
Dioxane solution—
Quantitatively dilute a volume of the Dioxane stock solution, accurately measured, with water to obtain a solution having a known concentration of about 0.5 mg of dioxane per mL.
Standard solution 1—
Transfer about 1.0 g of the substance under test, accurately weighed, to a 10-mL vial, and add 1.0 mL of N,N-dimethylacetamide, 0.1 mL of Ethylene oxide solution, and 0.1 mL of Dioxane solution. Close the vial, and mix to obtain a homogenous solution. Allow to stand at 90 for 45 minutes.
for 45 minutes.
Standard solution 2—
Transfer 0.1 mL of Ethylene oxide solution to a 10-mL vial, add 0.1 mL of a freshly prepared solution of acetaldehyde, containing about 10 mg of acetaldehyde per L, and add 0.1 mL of Dioxane solution. Close the vial, and mix to obtain a homogenous solution.
Test solution—
Transfer about 1.0 g of the substance under test, accurately weighed, to a 10-mL vial, and add 1.0 mL of N,N-dimethylacetamide and 0.2 mL of water. Close the vial, and mix to obtain a homogenous solution. Allow to stand at 90 for 45 minutes.
for 45 minutes.
Chromatographic system (see Chromatography  621
621 )—
[note—Headspace apparatus that automatically transfers a measured amount of headspace may be used.] The gas chromatograph is equipped with a flame-ionization detector and contains a 0.32-mm × 30-m glass or quartz capillary column bonded with a 1.0-µm layer of phase G1. The carrier gas is helium, flowing at a rate of about 1 mL per minute. The detector and injection port temperatures are maintained at 250
)—
[note—Headspace apparatus that automatically transfers a measured amount of headspace may be used.] The gas chromatograph is equipped with a flame-ionization detector and contains a 0.32-mm × 30-m glass or quartz capillary column bonded with a 1.0-µm layer of phase G1. The carrier gas is helium, flowing at a rate of about 1 mL per minute. The detector and injection port temperatures are maintained at 250 and 150
and 150 , respectively. The column temperature is programmed as follows. Initially it is maintained at 50
, respectively. The column temperature is programmed as follows. Initially it is maintained at 50 for 5 minutes after injection, then increased to 180
for 5 minutes after injection, then increased to 180 at a rate of 5
at a rate of 5 per minute, further increased to 230
per minute, further increased to 230 at a rate of 30
at a rate of 30 per minute, and maintained at this temperature for 5 minutes. Chromatograph the gaseous phase of Standard solution 2, and record the peak responses as directed for Procedure, adjusting the sensitivity of the system so that the peak heights of the two principal peaks in the chromatogram are not less than 15% of the full scale of the recorder: the relative retention times are about 0.94 for acetaldehyde and 1.0 for ethylene oxide; the resolution, R, between acetaldehyde and ethylene oxide is not less than 2.0; and the relative standard deviation for replicate injections is not more than 15.0%.
per minute, and maintained at this temperature for 5 minutes. Chromatograph the gaseous phase of Standard solution 2, and record the peak responses as directed for Procedure, adjusting the sensitivity of the system so that the peak heights of the two principal peaks in the chromatogram are not less than 15% of the full scale of the recorder: the relative retention times are about 0.94 for acetaldehyde and 1.0 for ethylene oxide; the resolution, R, between acetaldehyde and ethylene oxide is not less than 2.0; and the relative standard deviation for replicate injections is not more than 15.0%.
Procedure—
Using a heated, gas-tight gas chromatographic syringe, separately inject equal volumes (about 1 mL) of the gaseous headspace of Standard solution 1 and the Test solution into the chromatograph, record the chromatograms, and measure the peak responses: the mean areas of the ethylene oxide and dioxane peaks in the chromatogram obtained from the Test solution are not greater than half the mean areas of the corresponding peaks in the chromatogram obtained from Standard solution 1, equivalent to about 1 µg of ethylene oxide per g and 50 µg of dioxane per g. Calculate the concentration of ethylene oxide, in µg per g, in the test specimen taken by the formula:
CrU /(rS MU 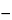 rU MS)
rU MS)
in which C is the concentration, in µg per mL, of ethylene oxide in Standard solution 1; rU and rS are the peak responses of ethylene oxide obtained from the Test solution and Standard solution 1, respectively; and MU and MS are the quantities, in g, of the substance under test taken to prepare the Test solution and Standard solution 1, respectively: not more than 1 µg per g is found. Calculate the concentration of dioxane, in µg per g, in the test specimen taken by the formula:
CD dU / 5(dS MU 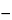 dU MS)
dU MS)
in which CD is the concentration, in µg, of dioxane in Standard solution 1; dU and dS are the peak responses of dioxane obtained from the Test solution and Standard solution 1, respectively; and MU and MS are as defined above: not more than 10 µg per g is found.
Limit of free glycerol—
Dissolve 1.20 g of the substance under test in 25 mL of methylene chloride, heating if necessary. Cool, and add 100 mL of water and 25.0 mL of periodic acid solution, prepared by dissolving 150 mg of periodic acid in 25 mL of water. Shake, and allow to stand for 30 minutes. Add 40 mL of potassium iodide solution, prepared by dissolving 3 g of potassium iodide in 40 mL of water, and allow to stand for 1 minute. Add 1 mL of starch TS, and titrate the liberated iodine with 0.1 M sodium thiosulfate. Perform a blank determination, and make any necessary correction (see Titrimetry  541
541 ). Each mL of 0.1 M sodium thiosulfate is equivalent to 2.3 mg of glycerol: not more than 5.0% is found.
). Each mL of 0.1 M sodium thiosulfate is equivalent to 2.3 mg of glycerol: not more than 5.0% is found.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Hong Wang, Ph.D.
Scientist 1-301-816-8351 |
(EM205) Excipient Monographs 2 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 1183
Pharmacopeial Forum: Volume No. 34(4) Page 1012