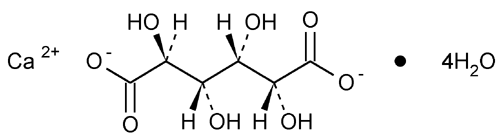
Calcium Saccharate
d-Glucaric acid, calcium salt (1:1) tetrahydrate.
Calcium d-glucarate (1:1), tetrahydrate
» Calcium Saccharate is the calcium salt of d-saccharic acid. It contains not less than 98.5 percent and not more than 102.0 percent of C6H8CaO8·4H2O.
Packaging and storage—
Preserve in well-closed containers.
USP Reference standards  11
11 —
—
USP Calcium Saccharate RS.
USP Calcium Saccharate RS.
Identification—
A:
Dissolve about 0.2 g in 10 mL of water by the addition of 2 mL of hydrochloric acid: the solution so obtained responds to the tests for Calcium  191
191 .
.
Specific rotation  781S
781S :
between +18.5
:
between +18.5 and +22.5
and +22.5 .
.
Test solution:
60 mg per mL, in 4.8 N hydrochloric acid that has been allowed to stand for 1 hour.
Chloride  221
221 —
A 0.50-g portion dissolved in 10 mL of water by the addition of 2 mL of nitric acid shows no more chloride than corresponds to 0.50 mL of 0.020 N hydrochloric acid (0.07%).
—
A 0.50-g portion dissolved in 10 mL of water by the addition of 2 mL of nitric acid shows no more chloride than corresponds to 0.50 mL of 0.020 N hydrochloric acid (0.07%).
Sulfate  221
221 —
A 0.50-g portion dissolved in 10 mL of water by the addition of 2 mL of hydrochloric acid shows no more sulfate than corresponds to 0.60 mL of 0.020 N sulfuric acid (0.12%).
—
A 0.50-g portion dissolved in 10 mL of water by the addition of 2 mL of hydrochloric acid shows no more sulfate than corresponds to 0.60 mL of 0.020 N sulfuric acid (0.12%).
Heavy metals, Method II  231
231 :
0.002%.
:
0.002%.
Sucrose and reducing sugars—
Dissolve 0.5 g in 10 mL of water by the addition of 2 mL of hydrochloric acid, and boil the solution for about 2 minutes. Cool, add 15 mL of sodium carbonate TS, allow to stand for 5 minutes, and filter. Add 5 mL of the clear filtrate to about 2 mL of alkaline cupric tartrate TS, and boil for 1 minute: no red precipitate is formed immediately.
Assay—
Dissolve about 600 mg of Calcium Saccharate, accurately weighed, in 150 mL of water with the aid of a sufficient volume of hydrochloric acid. While stirring, preferably with a magnetic stirrer, add about 30 mL of 0.05 M edetate disodium VS from a 50-mL buret. Add 15 mL of 1 N sodium hydroxide and 300 mg of hydroxy naphthol blue, and continue the titration to a blue endpoint. Each mL of 0.05 M edetate disodium is equivalent to 16.01 mg of C6H8CaO8·4H2O.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Robert H. Lafaver, B.A.
Scientist 1-301-816-8335 |
(EM105) Excipient Monographs 1 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 1772
Pharmacopeial Forum: Volume No. 27(6) Page 3261
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.