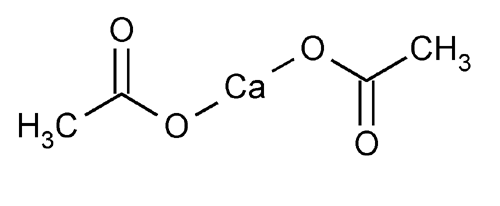
Calcium Acetate
» Calcium Acetate contains not less than 99.0 percent and not more than 100.5 percent of C4H6CaO4, calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
Labeling—
Where Calcium Acetate is intended for use in hemodialysis or peritoneal dialysis, it is so labeled.
pH  791
791 :
6.3 to 9.6, in a solution (1 in 20).
:
6.3 to 9.6, in a solution (1 in 20).
Water, Method I  921
921 :
not more than 7.0%, determined in a 0.7-g specimen, 20 mL of glacial acetic acid being added to the titration vessel in addition to the methanol.
:
not more than 7.0%, determined in a 0.7-g specimen, 20 mL of glacial acetic acid being added to the titration vessel in addition to the methanol.
Limit of fluoride—
Proceed as directed in the test for Limit of fluoride under Dibasic Calcium Phosphate: the limit is 0.005%.
Arsenic, Method I  211
211 :
3 ppm.
:
3 ppm.
Heavy metals, Method I  231
231 —
Prepare the Test Preparation as follows. Dissolve 0.8 g in 20 mL of water, add 3.0 mL of glacial acetic acid, dilute with water to 25 mL, and adjust with glacial acetic acid to a pH between 3.8 and 4.0, measured with a pH meter. Prepare the Monitor Preparation as directed for Test Preparation, 2.0 mL of Standard Lead Solution being added: the limit is 0.0025%.
—
Prepare the Test Preparation as follows. Dissolve 0.8 g in 20 mL of water, add 3.0 mL of glacial acetic acid, dilute with water to 25 mL, and adjust with glacial acetic acid to a pH between 3.8 and 4.0, measured with a pH meter. Prepare the Monitor Preparation as directed for Test Preparation, 2.0 mL of Standard Lead Solution being added: the limit is 0.0025%.
Lead  251
251 :
0.001%.
:
0.001%.
Chloride  221
221 —
A 1.0-g portion shows no more chloride than corresponds to 0.70 mL of 0.020 N hydrochloric acid (0.05%).
—
A 1.0-g portion shows no more chloride than corresponds to 0.70 mL of 0.020 N hydrochloric acid (0.05%).
Sulfate  221
221 —
A 0.25-g portion shows no more sulfate than corresponds to 0.15 mL of 0.020 N sulfuric acid (0.06%).
—
A 0.25-g portion shows no more sulfate than corresponds to 0.15 mL of 0.020 N sulfuric acid (0.06%).
Limit of nitrate—
Dissolve 1.0 g of it in 10 mL of water, add 5 mg of sodium chloride, 0.05 mL of indigo carmine TS, and, with stirring, 10 mL of nitrogen-free sulfuric acid: the blue color persists for not less than 10 minutes.
Limit of aluminum (where it is labeled as intended for parenteral use or for use in hemodialysis or peritoneal dialysis)—
pH 6.0 acetate buffer—
Dissolve 50 g of ammonium acetate in 150 mL of water, adjust with glacial acetic acid to a pH of 6.0, dilute with water to 250 mL, and mix.
Procedure—
Dissolve 1.0 g of it in 50 mL of water, and add 5 mL of pH 6.0 acetate buffer. Extract this solution with successive portions of 10, 10, and 5 mL of a 0.5% solution of 8-hydroxyquinoline in chloroform, combining the chloroform extracts in a 50-mL volumetric flask. Dilute the combined extracts with chloroform to volume, and mix (test solution). Prepare a Standard solution from a mixture of 2.0 mL of a solution containing 1.0 µg of Al per mL, prepared as directed for Standard Preparations under Aluminum  206
206 , 5 mL of pH 6.0 acetate buffer, and 48 mL of water, and extract as described above. Prepare a blank solution from a mixture of 50 mL of water and 5 mL of pH 6.0 acetate buffer, and extract as described above. Determine the fluorescence intensities of the test solution and the Standard solution in a fluorometer set at an excitation wavelength of 392 nm and an emission wavelength of 518 nm, using the blank solution to set the instrument to zero. The fluorescence of the test solution does not exceed that of the Standard solution (2 µg per g).
, 5 mL of pH 6.0 acetate buffer, and 48 mL of water, and extract as described above. Prepare a blank solution from a mixture of 50 mL of water and 5 mL of pH 6.0 acetate buffer, and extract as described above. Determine the fluorescence intensities of the test solution and the Standard solution in a fluorometer set at an excitation wavelength of 392 nm and an emission wavelength of 518 nm, using the blank solution to set the instrument to zero. The fluorescence of the test solution does not exceed that of the Standard solution (2 µg per g).
Barium (where it is labeled as intended for use in hemodialysis or peritoneal dialysis)—
Stock test solution—
Dissolve 50 g of it and 5 g of ammonium acetate in 200 mL of 1 N hydrochloric acid, and filter. The pH of this solution is between 4.5 and 5.5. [note—Cover the solution.]
Barium chloride solution—
Dissolve an accurately weighed quantity of anhydrous barium chloride in water to obtain a solution having a concentration of 0.758 mg per mL. This solution contains 500 µg of barium per mL.
Test solutions—
To four separate tubes add 1.0, 1.5, 2.0, and 2.5 mL of Barium chloride solution. To each tube add a sufficient volume of the Stock test solution to bring the volume to 40 mL.
Standard solution—
To a fifth tube add 1 g of ammonium acetate, 2 mL of 1 N hydrochloric acid, 3.0 mL of Barium chloride solution, and sufficient water to bring the volume to 40 mL.
Ammonium sulfate solution—
Use a solution of ammonium sulfate (1 in 10).
Procedure—
To the Test solutions and the Standard solution add, with brisk stirring, 3.0 mL of Ammonium sulfate solution, and allow to stand for 20 minutes. The Test solutions containing 1.0 and 1.5 mL of Barium chloride solution remain clear or are only faintly turbid. The Test solution containing 2.0 mL of Barium chloride solution is not more turbid than the Standard solution.
Limit of magnesium (where it is labeled as intended for use in hemodialysis or peritoneal dialysis)—
[note—The Standard preparation and the test solutions may be modified, if necessary, to obtain solutions of suitable concentrations, adaptable to the linear or working range of the instrument.]
Standard preparation—
Dissolve an accurately weighed quantity of magnesium oxide in 1 N nitric acid to obtain a solution having a concentration of 1.516 mg per mL. This solution contains 1000 µg of magnesium per mL. Dilute an accurately measured volume of this solution quantitatively, and stepwise if necessary, with water to obtain a solution containing 5.0 µg of magnesium per mL.
Test preparation—
Transfer 200 mg of Calcium Acetate to a 100-mL volumetric flask, dissolve in and dilute with water to volume, and mix.
Procedure—
To three separate 25-mL volumetric flasks add 0, 2.0, and 4.0 mL of the Standard preparation. To each flask add 20.0 mL of the Test preparation, dilute with water to volume, and mix. These test solutions contain, respectively, 0, 0.4, and 0.8 µg per mL of magnesium from the Standard preparation. Concomitantly determine the absorbances of the test solutions at the magnesium emission line at 285.2 nm with a suitable atomic absorption spectrophotometer (see Spectrophotometry and Light-Scattering  851
851 ) equipped with an air–acetylene flame, using water as the blank. Plot the absorbances of the test solutions versus their contents of magnesium, in µg per mL, as furnished by the Standard preparation, draw the straight line best fitting the three points, and extrapolate the line until it intercepts the concentration axis. From the intercept determine the amount, in µg, of magnesium in each mL of the test solution containing 0 mL of the Standard preparation. Calculate the percentage of magnesium in the specimen by multiplying this value by 0.0625: the limit is 0.05%.
) equipped with an air–acetylene flame, using water as the blank. Plot the absorbances of the test solutions versus their contents of magnesium, in µg per mL, as furnished by the Standard preparation, draw the straight line best fitting the three points, and extrapolate the line until it intercepts the concentration axis. From the intercept determine the amount, in µg, of magnesium in each mL of the test solution containing 0 mL of the Standard preparation. Calculate the percentage of magnesium in the specimen by multiplying this value by 0.0625: the limit is 0.05%.
Limit of potassium (where it is labeled as intended for use in hemodialysis or peritoneal dialysis)—
[note—The Standard preparation and the test solutions may be modified, if necessary, to obtain solutions of suitable concentrations, adaptable to the linear or working range of the instrument.]
Standard preparation—
Transfer 5.959 g of potassium chloride, previously dried at 105 for 2 hours and accurately weighed, to a 250-mL volumetric flask, dilute with water to volume, and mix. This solution contains 12.5 mg of potassium per mL. Dilute an accurately measured volume of this solution quantitatively, and stepwise if necessary, with water to obtain a solution containing 31.25 µg of potassium per mL.
for 2 hours and accurately weighed, to a 250-mL volumetric flask, dilute with water to volume, and mix. This solution contains 12.5 mg of potassium per mL. Dilute an accurately measured volume of this solution quantitatively, and stepwise if necessary, with water to obtain a solution containing 31.25 µg of potassium per mL.
Test preparation—
Transfer 1.25 g of Calcium Acetate to a 100-mL volumetric flask, dissolve in and dilute with water to volume, and mix.
Procedure—
To three separate 25-mL volumetric flasks add 0, 2.0, and 4.0 mL of the Standard preparation. To each flask add 20.0 mL of the Test preparation, dilute with water to volume, and mix. These test solutions contain, respectively, 0, 2.5, and 5.0 µg per mL of potassium from the Standard preparation. Concomitantly determine the absorbances of the test solutions at the potassium emission line at 766.7 nm with a suitable atomic absorption spectrophotometer (see Spectrophotometry and Light-Scattering  851
851 ) equipped with an air–acetylene flame, using water as the blank. Plot the absorbances of the test solutions versus their contents of potassium, in µg per mL, as furnished by the Standard preparation, draw the straight line best fitting the three points, and extrapolate the line until it intercepts the concentration axis. From the intercept determine the amount, in µg, of potassium in each mL of the test solution containing 0 mL of the Standard preparation. Calculate the percentage of potassium in the specimen by multiplying this value by 0.01: the limit is 0.05%.
) equipped with an air–acetylene flame, using water as the blank. Plot the absorbances of the test solutions versus their contents of potassium, in µg per mL, as furnished by the Standard preparation, draw the straight line best fitting the three points, and extrapolate the line until it intercepts the concentration axis. From the intercept determine the amount, in µg, of potassium in each mL of the test solution containing 0 mL of the Standard preparation. Calculate the percentage of potassium in the specimen by multiplying this value by 0.01: the limit is 0.05%.
Limit of sodium (where it is labeled as intended for use in hemodialysis or peritoneal dialysis)—
[note—The Standard preparation and the test solutions may be modified, if necessary, to obtain solutions of suitable concentrations, adaptable to the linear or working range of the instrument.]
Standard preparation—
Transfer 6.355 g of sodium chloride, previously dried at 105 for 2 hours and accurately weighed, to a 250-mL volumetric flask, dilute with water to volume, and mix. This solution contains 10.0 mg of sodium per mL. Dilute an accurately measured volume of this solution quantitatively, and stepwise if necessary, with water to obtain a solution containing 250 µg of sodium per mL.
for 2 hours and accurately weighed, to a 250-mL volumetric flask, dilute with water to volume, and mix. This solution contains 10.0 mg of sodium per mL. Dilute an accurately measured volume of this solution quantitatively, and stepwise if necessary, with water to obtain a solution containing 250 µg of sodium per mL.
Test preparation—
Transfer 1.0 g of Calcium Acetate to a 100-mL volumetric flask, dissolve in and dilute with water to volume, and mix.
Procedure—
To three separate 25-mL volumetric flasks add 0, 2.0, and 4.0 mL of the Standard preparation. To each flask add 20.0 mL of the Test preparation, dilute with water to volume, and mix. These test solutions contain, respectively, 0, 20.0, and 40.0 µg per mL of sodium from the Standard preparation. Concomitantly determine the absorbances of the test solutions at the sodium emission line at 589.0 nm with a suitable atomic absorption spectrophotometer (see Spectrophotometry and Light-Scattering  851
851 ) equipped with an air–acetylene flame, using water as the blank. Plot the absorbances of the test solutions versus their contents of sodium, in µg per mL, as furnished by the Standard preparation, draw the straight line best fitting the three points, and extrapolate the line until it intercepts the concentration axis. From the intercept determine the amount, in µg, of sodium in each mL of the test solution containing 0 mL of the Standard preparation. Calculate the percentage of sodium in the specimen by multiplying this value by 0.0125: the limit is 0.5%.
) equipped with an air–acetylene flame, using water as the blank. Plot the absorbances of the test solutions versus their contents of sodium, in µg per mL, as furnished by the Standard preparation, draw the straight line best fitting the three points, and extrapolate the line until it intercepts the concentration axis. From the intercept determine the amount, in µg, of sodium in each mL of the test solution containing 0 mL of the Standard preparation. Calculate the percentage of sodium in the specimen by multiplying this value by 0.0125: the limit is 0.5%.
Limit of strontium (where it is labeled as intended for use in hemodialysis or peritoneal dialysis)—
[note—The Standard preparation and the test solutions may be modified, if necessary, to obtain solutions of suitable concentrations, adaptable to the linear or working range of the instrument.]
Standard preparation—
Dissolve an accurately weighed quantity of strontium acetate in water to obtain a solution having a concentration of 2.45 mg per mL. This solution contains 1000 µg of strontium per mL. Dilute an accurately measured volume of this solution quantitatively, and stepwise if necessary, with water to obtain a solution containing 50.0 µg of strontium per mL.
Test preparation—
Transfer 2.0 g of Calcium Acetate to a 100-mL volumetric flask, dissolve in and dilute with water to volume, and mix.
Procedure—
To three separate 25-mL volumetric flasks add 0, 2.0, and 4.0 mL of the Standard preparation. To each flask add 20.0 mL of the Test preparation, dilute with water to volume, and mix. These test solutions contain, respectively, 0, 4.0, and 8.0 µg per mL of strontium from the Standard preparation. Concomitantly determine the absorbances of the test solutions at the strontium emission line at 460.7 nm with a suitable atomic absorption spectrophotometer (see Spectrophotometry and Light-Scattering  851
851 ) equipped with a nitrous oxide–acetylene flame, using water as the blank. Plot the absorbances of the test solutions versus their contents of strontium, in µg per mL, as furnished by the Standard preparation, draw the straight line best fitting the three points, and extrapolate the line until it intercepts the concentration axis. From the intercept determine the amount, in µg, of strontium in each mL of the test solution containing 0 mL of the Standard preparation. Calculate the percentage of strontium in the specimen by multiplying this value by 0.00625: the limit is 0.05%.
) equipped with a nitrous oxide–acetylene flame, using water as the blank. Plot the absorbances of the test solutions versus their contents of strontium, in µg per mL, as furnished by the Standard preparation, draw the straight line best fitting the three points, and extrapolate the line until it intercepts the concentration axis. From the intercept determine the amount, in µg, of strontium in each mL of the test solution containing 0 mL of the Standard preparation. Calculate the percentage of strontium in the specimen by multiplying this value by 0.00625: the limit is 0.05%.
Readily oxidizable substances—
Dissolve 2.0 g of it in 100 mL of boiling water, add a few glass beads, 6 mL of 10 N sulfuric acid, and 0.3 mL of 1 N potassium permanganate, mix, boil gently for 5 minutes, and allow the precipitate to settle: the pink color in the supernatant is not completely discharged.
Assay—
Dissolve about 300 mg of Calcium Acetate, accurately weighed, in 150 mL of water containing 2 mL of 3 N hydrochloric acid. While stirring, preferably with a magnetic stirrer, add about 30 mL of 0.05 M edetate disodium VS from a 50-mL buret. Add 15 mL of 1 N sodium hydroxide and 300 mg of hydroxy naphthol blue, and continue the titration to a blue endpoint. Each mL of 0.05 M edetate disodium is equivalent to 7.909 mg of C4H6CaO4.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Daniel K. Bempong, Ph.D.
Senior Scientist 1-301-816-8345 |
(MDPS05) Monograph Development-Pulmonary and Steroids |
USP32–NF27 Page 1752
Pharmacopeial Forum: Volume No. 27(6) Page 3254
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.