| 再评价结果通知号及日期 | 11 2001/12/25 |
|---|---|
| 药效分类 | 613 Antibiotic preparations acting mainly on gram-positive, gram-negativebacteria |
| 商品名 | SUMACEF Cap. 250 |
| 活性成分 | Cefadroxil |
| 活性成分(日文) | セファドロキシル |
| 剂型 | Capsules |
| 规格 | 250mg |
| 特性 | Immediate-release |
| 转速 | Paddle Method,50rpm |
| 溶出液 | 基准液:4 其他:1.2 6.8 water |
| 溶出规格 | 90minutes:Not less than 80%(UV method) |
| 溶出曲线测定例 | 成分名称:セファドロキシル 英文名:Cefadroxil 含量:250mg 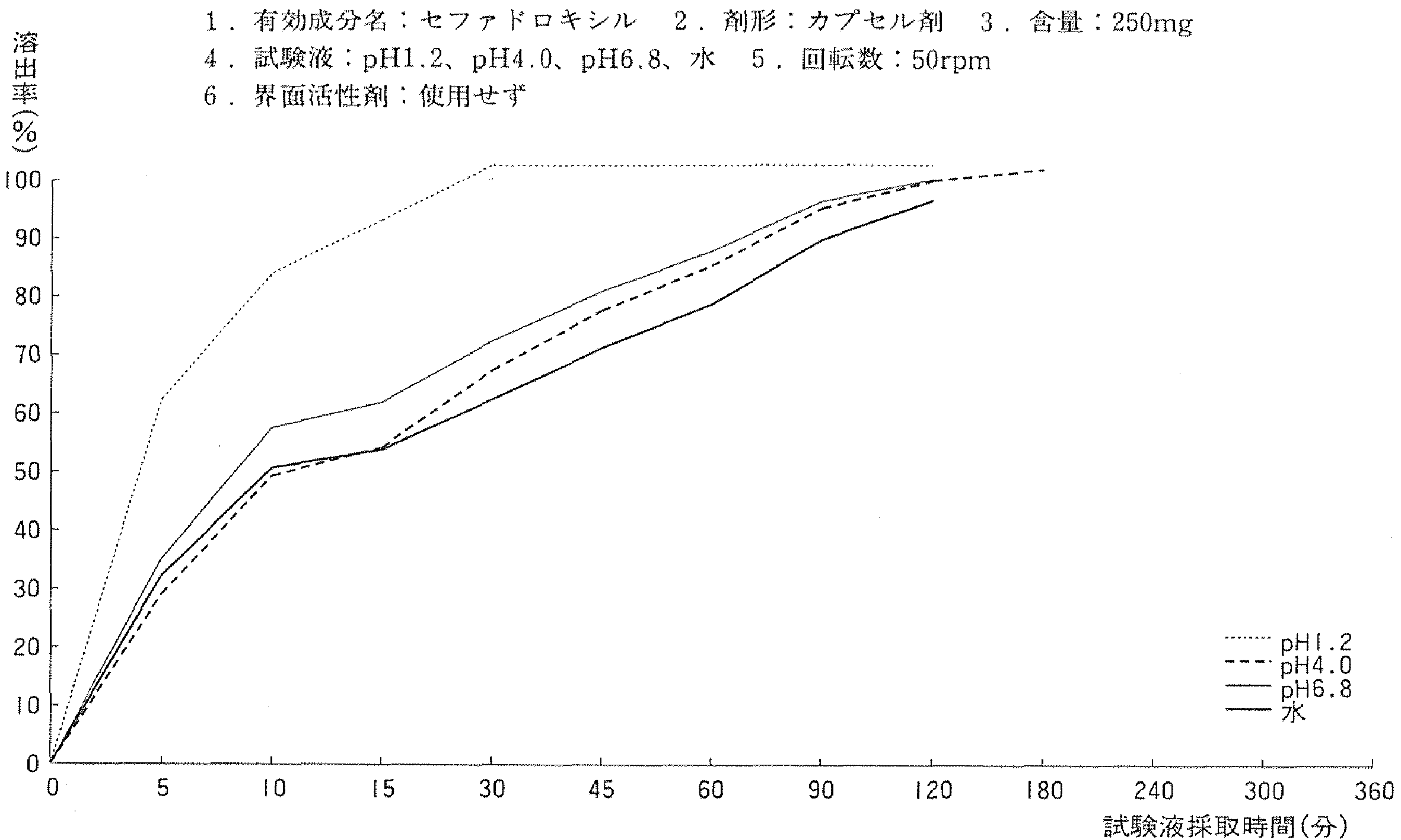 |