| 物质名称 | LEVONORGESTREL BUTYRATE |
|---|---|
| 异名/同义词 | (8R,9S,10R,13S,14S,17R)-13-ETHYL-17-ETHYNYL-3-OXO-2,3,6,7,8,9,10,11,12,13,14,15,16,17-TETRADECAHYDRO-1H-CYCLOPENTA(A)PHENANTHREN-17-YL BUTYRATE 13-ETHYL-17.ALPHA.-HYDROXY-18,19-DINORPREGN-4-EN-20-YN-3-ONE BUTYRATE 18,19-DINORPREGN-4-EN-20-YN-3-ONE, 13-ETHYL-17-(1-OXOBUTOXY)-, (17.ALPHA.)- LEVONORGESTREL BUTANOATE LEVONORGESTREL BUTYRATE |
| CAS登记号 | 86679-33-6 |
| 物质唯一标识(Unique Ingredient Identifier) | L929CBB126 |
| 分子式 | C25H34O3 |
| 国际化合物标识(International Chemical Identifier,InChI) | GPKLGCALNRZIDS-AYEDEZQKSA-N |
| SMILES | [C@]12([C@@](C#C)(OC(=O)CCC)CC[C@]1([C@]3([C@]([C@@]4(C(=CC(=O)CC4)CC3)[H])([H])CC2)[H])[H])CC |
| 分子结构式 | 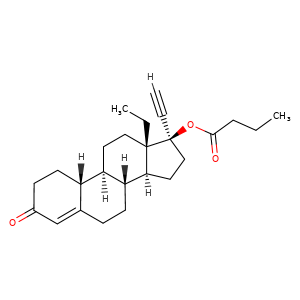 |
| 欧洲化学品管理局注册号(ECHA) | 289-270-0 |
| 成分类型 | INGREDIENT SUBSTANCE / chemical |
| 关联数据 | |
| 扩展资源 |