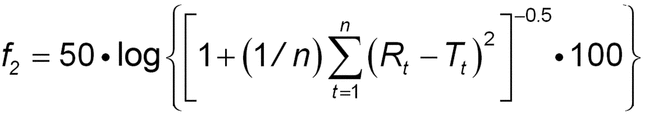PURPOSE
This chapter provides quality attributes for products with approved labeling indicating that the tablets can be split to produce multiple portions that have an accurate fractional dose (labeled as functionally scored). The label claim of the split portions should be a simple fractional part of the claim for the intact tablet based on the number of scores and the size of the split portion (e.g., one-half, one-third, or one-quarter). At the time of splitting, the intact tablets should conform to the monograph specification. With the exception of dose, each split portion from tablets labeled as having a functional score are expected to conform to the quality attributes of the whole tablets. The split portions resulting from subdividing a functionally scored tablet should conform to the tests for Splitting Tablets with Functional Scoring and Dissolution or Disintegration given in this chapter.
SCOPE
This chapter applies to tablets labeled as having a functional score and to the split portions that represent any labeled fraction of the whole functionally scored tablet dose. Tablets should be split as part of the test procedure and the storage conditions for the split portions should be defined in the test procedure. For Dissolution or Disintegration testing, analysts should use only split portions from tablets determined to be acceptable by the Splitting Tablets with Functional Scoring test.
SPLITTING TABLETS WITH FUNCTIONAL SCORING
Test Procedure
- Take a random sample of 30 intact tablets, and proceed as follows.
- Accurately weigh each tablet, and record its weight.
- For each intact tablet, determine the expected weight of the split portions by dividing the whole-tablet weight by the designated number of split portions indicated on the labeling.
- Split each tablet by hand (without mechanical assistance) into the designed number of split portions, and weigh each split portion.
- For each tablet, determine the percent of the expected weight in each split portion.
An acceptable tablet breaks into the designed number of segments, and each split portion has NLT 75% and NMT 125% of the expected weight of the split tablet portion. [Note—Set aside split tablet portions derived from acceptable tablets for subsequent testing for dissolution or disintegration. ]
Acceptance criteria: NLT 28 of the 30 tablets are acceptable.
DISSOLUTION
Use split portions from tablets that are acceptable according to the Splitting Tablets with Functional Scoring test.
Immediate-Release Tablets
Dissolution for immediate-release tablets is performed at the S2 stage (see Dissolution  711
711 ). Test 12 split tablet portions according to the specified Medium, Apparatus, Times, and Analysis. The average of the 12 results is NLT Q, and no result is less than Q – 15%.
). Test 12 split tablet portions according to the specified Medium, Apparatus, Times, and Analysis. The average of the 12 results is NLT Q, and no result is less than Q – 15%.
Extended-Release Tablets
Perform dissolution testing of split tablet portions from extended-release tablets by one of the two alternative procedures. The procedure to be used is specified in the monograph.
Procedure 1 (Procedure for Extended-Release Dosage Forms, Dissolution  711
711 ): Individually test 12 split tablet portions and 12 intact tablets.
): Individually test 12 split tablet portions and 12 intact tablets.
Medium, Apparatus, and Analysis: As given in the monograph following the appropriate test number found on the labeling. Dissolution profile test time points are determined as follows. From the appropriate dissolution test in the monograph, use the time points given. At a minimum, use three time points with no more than one time point where the results exceed 85% dissolved.
Calculate the similarity factor (f2) for the intact-tablet results and the split-tablet portion results:
Rt = cumulative percentage of the labeled drug dissolved at each of the selected n time points of the intact tablets
Tt = cumulative percentage of the labeled drug dissolved at each of the selected n time points of the split tablet portions
Acceptance criteria: The calculated f2 is NLT 50 (acceptable range: 50–100).
Procedure 2 (Procedure for Extended-Release Dosage Forms, Dissolution  711
711 ): Use a split-tablet portion as the dosage unit. Individually test 12 dosage units.
): Use a split-tablet portion as the dosage unit. Individually test 12 dosage units.
Medium, Apparatus, Times, and Analysis: As given in the monograph following the appropriate test number found on the labeling.
Acceptance criteria: The percentages of the labeled amount released at the times specified conform to the L2 level criteria of Acceptance Table 2 in  711
711 .
.
DISINTEGRATION
Disintegration testing is necessary only when used as a surrogate for dissolution testing as specified in the monograph. Follow the procedure using split portions from tablets that are acceptable according to the Splitting Tablets with Functional Scoring test as the dosage unit (see Disintegration  701
701 ).
).
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | William E. Brown
Senior Scientific Liaison (301) 816-8380 |
(GCDF2010) General Chapters - Dosage Forms |
USP38–NF33 Page 485
Pharmacopeial Forum: Volume No. 39(4)