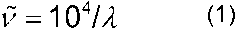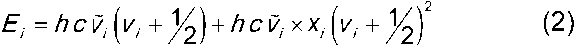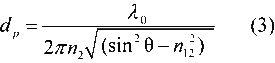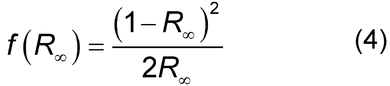Add the following:
PRINCIPLES OF MID-INFRARED SPECTROSCOPY
Mid-infrared (mid-IR) spectroscopy involves measurement of the absorption of electromagnetic radiation
 1 (which corresponds to the wavelength range of 2.5–25 µm) caused by the promotion of molecules from the ground state of their vibrational modes to an excited vibrational state. The most commonly used parameter to denote the energy of the transitions is the wavenumber, i.e., the number of waves per centimeter. The wavelength,
1 (which corresponds to the wavelength range of 2.5–25 µm) caused by the promotion of molecules from the ground state of their vibrational modes to an excited vibrational state. The most commonly used parameter to denote the energy of the transitions is the wavenumber, i.e., the number of waves per centimeter. The wavelength, 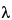 (µm), and wavenumber,
(µm), and wavenumber,  (cm
(cm 1), of radiation are related by the expression:
1), of radiation are related by the expression:
The mid-IR spectrum extends from 4000 cm 1 (2.5 µm) to 400 cm
1 (2.5 µm) to 400 cm 1 (25 µm). Molecules can move in a certain number of vibrational modes. The energy of mode, i, is given by:
1 (25 µm). Molecules can move in a certain number of vibrational modes. The energy of mode, i, is given by:
h = Planck’s constant
c = velocity of light
vi = vibrational quantum number of this mode
xi = so-called anharmonicity constant
The strongest bands in the mid-IR spectrum are caused by fundamental transitions from the ground state of a given mode (vi = 0) to its first excited vibrational state (vi = 1), although weaker overtone and combination bands also are observed in the spectrum. Overtone bands are caused by the promotion of molecules from their ground state to their second and higher vibrational states (vi = 2, 3, etc.). Overtones are observed only for those modes for which xi is non-zero. Combination bands are caused by the simultaneous promotion of molecules to two excited vibrational states.
Vibrational modes involve the motion of all atoms of the molecule. Many modes involve only large-amplitude vibrations of the atoms in localized regions of the molecule, and the remaining atoms are largely unaffected. When molecules contain a certain functional group, the transitions often occur in narrow spectral ranges. In this case, the wavenumbers at which these transitions occur are known as group frequencies. When a vibrational mode involves atomic motions of more than just a few atoms, the frequencies occur over wider spectral ranges and are not characteristic of a particular functional group. Instead, they are more characteristic of the molecules as a whole. Such bands are known as fingerprint bands. All strong bands that absorb at wavenumbers above 1500 cm 1 are group frequencies. Strong bands that absorb below 1500 cm
1 are group frequencies. Strong bands that absorb below 1500 cm 1 can either be group frequencies or fingerprint bands. Thus, not all strong bands in the IR spectrum of a given molecule can be attributed to the presence of a particular functional group.
1 can either be group frequencies or fingerprint bands. Thus, not all strong bands in the IR spectrum of a given molecule can be attributed to the presence of a particular functional group.
The motion of atoms during a particular vibrational mode, i, is characterized by the normal coordinate, Qi. The intensity of fundamental bands is governed by the square of the change in dipole moment, µ, during the vibrational cycle (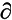 µ/
µ/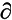 Qi)2. Thus vibrational transitions of modes involving polar groups, such as C–O, C=O, O–H, N–H, and C–F, typically give rise to strong bands in the spectrum. Where (
Qi)2. Thus vibrational transitions of modes involving polar groups, such as C–O, C=O, O–H, N–H, and C–F, typically give rise to strong bands in the spectrum. Where (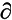 µ/
µ/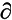 Qi) is small, transitions are weak. When the symmetry of a molecule leads to the condition that (
Qi) is small, transitions are weak. When the symmetry of a molecule leads to the condition that (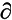 µ/
µ/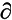 Qi) = 0 for a certain mode, the band corresponding to this mode does not appear in the IR spectrum. Overtone and combination bands are always weaker than the fundamental modes from which they are derived.
Qi) = 0 for a certain mode, the band corresponding to this mode does not appear in the IR spectrum. Overtone and combination bands are always weaker than the fundamental modes from which they are derived.
For functional groups that have the form XY2 (such as –CH2, –NH2, –NO2, and –SO2) and XY3 (such as –CH3 and –NH3+), first-order coupling can occur so that the mode is split into a symmetrical mode (where both the Y atoms move to and from the X atom with the same phase) and an antisymmetrical mode (where one Y atom moves 180 out of phase with the other). The amount by which the two bands are split depends on the Y–X–Y angle. The more closely this angle approaches 180
out of phase with the other). The amount by which the two bands are split depends on the Y–X–Y angle. The more closely this angle approaches 180 , the greater the splitting. Thus, for example, for the symmetric and antisymmetric stretching modes of ketenes and isocyanates where the angle is approximately 180
, the greater the splitting. Thus, for example, for the symmetric and antisymmetric stretching modes of ketenes and isocyanates where the angle is approximately 180 , the splitting can approach 1000 cm
, the splitting can approach 1000 cm 1, whereas for CH2 and CH3 groups where the angle is approximately 108
1, whereas for CH2 and CH3 groups where the angle is approximately 108 , the splitting is on the order of 100 cm
, the splitting is on the order of 100 cm 1.
1.
Second-order coupling, sometimes known as Fermi resonance, occurs when an overtone or combination band happens to occur at the same (or near coincident) wavenumber as a fundamental mode of the molecule that involves motion of the same atoms. In this case, the overtone or combination borrows intensity from the fundamental band, and the two bands split apart by as much as 40 cm 1. The closer the bands that interact in this manner, the greater the splitting and the closer the intensity for the resulting bands.
1. The closer the bands that interact in this manner, the greater the splitting and the closer the intensity for the resulting bands.
SAMPLING PROCEDURES
Mid-IR spectra can be measured by transmission, external reflection, internal reflection [often called attenuated total reflection (ATR)], diffuse reflection, and photoacoustic spectroscopy. Each of the major approaches is presented below.
Transmission Measurements
The alkali halide disk and mulling procedures are the traditional mid-IR transmission sample presentation methods for materials that are in the form of a finely divided powder, as is the case for many drug substances and excipients. During preparation of a sample suitable for IR spectroscopy, the powdered material is uniformly dispersed throughout either the alkali halide or mulling agent, which acts as a support matrix for the analyte. Other procedures by which transmission spectra can be acquired include the use of solutions and compression cells. Neat compounds can be examined in a compression cell, as a self-supporting film (for polymers), as a capillary film between the IR-transparent cell windows (for liquids and semisolids), and as a gas.
The ratio of the single-beam spectrum of the sample and an appropriate background spectrum at a given wavenumber,  , is known as the transmittance, T-(
, is known as the transmittance, T-( ). A transmittance spectrum is the direct output of most prism or grating spectrophotometers. For spectra measured on a Fourier transform-IR (FT-IR) spectrophotometer, the two single-beam spectra are measured at different times and are ratioed subsequently. An appropriate background spectrum should be measured. For
). A transmittance spectrum is the direct output of most prism or grating spectrophotometers. For spectra measured on a Fourier transform-IR (FT-IR) spectrophotometer, the two single-beam spectra are measured at different times and are ratioed subsequently. An appropriate background spectrum should be measured. For
Because transmission spectra of nonscattering samples obey the Beer–Lambert Law (usually abbreviated as Beer’s Law), the transmittance commonly is converted to absorbance, A ( ), i.e., log10 1/T (
), i.e., log10 1/T ( ). Beer’s Law states that the absorbance of component i at wavenumber, Ai (
). Beer’s Law states that the absorbance of component i at wavenumber, Ai ( ), is the product of the absorptivity of i at that wavenumber, ai (
), is the product of the absorptivity of i at that wavenumber, ai ( ), the path length of the sample, b, and the concentration of i, ci. The measured absorbance of a mixture at each wavenumber is the sum of the absorbances of each component of the mixture.
), the path length of the sample, b, and the concentration of i, ci. The measured absorbance of a mixture at each wavenumber is the sum of the absorbances of each component of the mixture.
Potassium Bromide (KBr) Disks
Commercial presses and dies in a range of diameters are available for the preparation of alkali halide and similar disks. The most common diameter of KBr disks is 13 mm, but mini-disks with a diameter as small as 0.5 mm can be prepared using commercially available presses. Follow the manufacturer’s recommended procedures for operating the disk-making accessory and the press.
Typically, the weight ratio of sample to alkali halide is on the order of 1 part of the sample to 100–400 parts of potassium bromide. An optimal procedure for preparing a 13-mm diameter disk is to pregrind the sample to a fine particle size in
 1. Faulty, unsatisfactory, or poor-quality disks may be a consequence of inadequate or excessive grinding, moisture/humidity, or impurities in the dispersion medium.
1. Faulty, unsatisfactory, or poor-quality disks may be a consequence of inadequate or excessive grinding, moisture/humidity, or impurities in the dispersion medium.
Mulls
To prepare a mull, homogeneously distribute the finely divided powder sample in a thin layer of a viscous liquid that is semi-transparent to mid-IR radiation and has a refractive index closely matched to that of the sample. The prepared mull is sandwiched between a pair of mid-IR–transparent windows, and a transmission spectrum of the mull preparation is recorded. The mull, which should have the consistency of a paste, is formed in such a manner as to minimize radiation scattering effects (radiation scatter from particles is worse when there is greater mismatch between the refractive index of the dispersant and surrounding medium). The sandwich can be clamped together in a mull cell. Commercial mull cells are available for both macro- and micro-preparations.
The most widely used mulling agent for the mid-IR region is a saturated hydrocarbon mineral oil (liquid paraffin, Nujol). This material has strong absorption bands of 3000–2800 cm 1 and of 1500–1340 cm
1 and of 1500–1340 cm 1, and a weaker band at 720 cm
1, and a weaker band at 720 cm 1 that may obscure the absorption bands of the sample in these regions. In this case, a mull may require preparation in a chemically different oil. This can be achieved by the use of a perhalogenated oil mulling agent such as
1 that may obscure the absorption bands of the sample in these regions. In this case, a mull may require preparation in a chemically different oil. This can be achieved by the use of a perhalogenated oil mulling agent such as
For the preparation of a mineral oil mull, the particle size of the sample must be reduced to below that of the shortest wavelength of the interrogating radiation (2.5 µm) in order to minimize light-scattering effects that decrease spectral contrast and cause band distortions. The spectrum from a coarse powder or one that is poorly ground will show a high degree of scatter that is manifested as a sloping baseline that decreases toward shorter wavelengths (higher wavenumbers). Also, a coarser powder will increase the Christiansen effect, which is caused by reflection from the interface between materials of different refractive index. The refractive index of materials with strong absorption bands varies in a way that is similar to the first derivative of the profile of the absorption band—a phenomenon that is referred to as anomalous dispersion. The Christiansen effect is manifested as a transmission increase on the short-wavelength (high-wavenumber) side of an absorption band with a concomitant decrease on the longer wavelength side. Furthermore, a coarse, poorly dispersed powder can lead to a severely distorted mid-IR spectrum in which the relative intensity of the weaker bands is enhanced and the intensity of the more intense bands in the spectrum appears weaker and distorted. These effects are a consequence of radiation that has reached the detector but has not been transmitted through a representative sample of the analyte. Similar effects can be seen in the spectra of poorly prepared KBr disks.
During the preparation of mulls and KBr disks, some work is done on the analyte, either in the form of grinding, mixing, or pressing, and consequently there is the potential to induce solid-state form transformations. Although laboratory mechanical mills can produce powders with a small particle size, hand grinding using a mortar and pestle usually achieves better control and less aggressive processing for organic pharmaceutical materials. Practitioners generally accept that of the two procedures, the mull procedure is the less aggressive and is less prone to induce solid-state form changes such as changes in the crystallinity (polymorphism) or changes in the hydration or solvation state (pseudopolymorphism). The KBr disk procedure does, however, have advantages over the mull presentation method because potassium bromide exhibits no absorption bands above 400 cm 1 (neglecting any adsorbed water or impurities) and is better adapted to micro-sample preparations. When the sample is a salt, as is frequently the case for active pharmaceutical ingredients (APIs), ion exchange can occur between the analyte and alkali halide, and the sample is better prepared as a mull.
1 (neglecting any adsorbed water or impurities) and is better adapted to micro-sample preparations. When the sample is a salt, as is frequently the case for active pharmaceutical ingredients (APIs), ion exchange can occur between the analyte and alkali halide, and the sample is better prepared as a mull.
Compression Cells
The use of a compression cell has become a popular sampling procedure for recording a mid-IR transmission spectrum of a small or limited-quantity solid sample such as a single particle of an API or excipient, a contaminant such as a short length of fiber, or a small fragment from a packaging material. This is particularly the case for investigations using an IR microscope system. Type IIa diamonds are quite transparent over much of the mid-IR region, although they exhibit fairly strong absorption between approximately 2000 and 2400 cm 1. Because of the high strength of diamond, it is commonly used as the window material of compression cells. The sample is placed between the diamond windows of the cell, the cell is then tightened, and the sample thickness is reduced to an optimum for a transmission measurement. The compressed sample can be examined while it is contained within the compression cell.
1. Because of the high strength of diamond, it is commonly used as the window material of compression cells. The sample is placed between the diamond windows of the cell, the cell is then tightened, and the sample thickness is reduced to an optimum for a transmission measurement. The compressed sample can be examined while it is contained within the compression cell.
Self-Supported Polymer Films
The mid-IR transmission spectrum of many polymers used as packaging materials can be recorded from samples prepared as thin self-supporting films. Films of appropriate thickness can be prepared by, for example, hot compression moulding a sample or microtoming a thin section from a sample. Soft and low-melting solids that do not crystallize when cooled can be prepared either as a thin layer sandwiched between two mid-IR–transparent windows by gently warming the sample or from the melt. Thin films from some materials can be cast from solution onto an IR-transparent window.
Capillary Films
Nonvolatile liquids can be examined neat in the form of a thin layer sandwiched between two matching windows that are transparent to IR radiation. The liquid layer must be free of bubbles and must completely cover the diameter of the IR beam focused onto the sample.
Liquids and Solutions in Transmission Cells
For the examination of liquid and solution samples, transmission cell assemblies that comprise a pair of windows constructed of mid-IR–transparent materials such as potassium bromide spacers, filling ports, and a holder are available commercially in both macro- and micro-sample configurations. They can be sealed, semi-permanent, or flow-through. A wide range of standard thickness spacers and window materials is available. For laboratory applications, spacers are typically formed from lead, poly(tetrafluoroethylene), or poly(ethylene terephthalate) and can be supplied, depending on spacer materials, in standard thickness path lengths from approximately 6 µm to 1 mm or larger.
The optimum path length required for examining a particular liquid or solution usually must be determined empirically and depends on its absorption characteristics and whether the application is qualitative or quantitative. In some instances for qualitative work, transmission cells can be replaced with disposable porous media such as polyethylene or poly(tetrafluoroethylene) mounted on a suitable backing.
Gases
Mid-IR transmission cells for static or flow-through gas and vapor sampling are available in a wide range of materials to suit the application, from laboratory to process scale. In the laboratory, the traditional gas cell has been a 10-cm long cylinder made from borosilicate glass or stainless steel with an aperture of about 40 mm at each end. Each open end is covered with an end cap that contains one of a pair of mid-IR–transparent windows constructed from, for example, potassium bromide, zinc selenide, or calcium fluoride. The cell body is fitted with appropriate inlet and outlet ports.
 or greater, and their pressure ratings can range from vacuum to more than 50 atmospheres.
or greater, and their pressure ratings can range from vacuum to more than 50 atmospheres.
Attenuated Total Reflection Spectroscopy
Attenuated total reflection (ATR) spectroscopy, alternatively known as internal reflection spectroscopy or evanescent wave spectroscopy, has become a widely used procedure. This is largely a consequence of a new generation of simple-to-use single-reflection accessories.
ATR spectroscopy relies on the optical property that radiation passing through a medium of high refractive index, n2 [the optically dense medium, also known as the internal reflection element (IRE)], at an angle of incidence greater than the critical angle will be totally internally reflected at a boundary in contact with a material of lower refractive index,
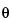 C, is given by n1/n2. The electric field of the radiation penetrates a short distance into the optically rare medium. The intensity of this electric field, which is known as the evanescent wave, is confined within the vicinity of the surface of the denser medium. Its intensity decreases exponentially with distance, normal to the surface, into the optically rare medium. It can, therefore, be envisaged as penetrating the surface layer of the rarer medium. The depth of penetration, dp, is a convenient comparative term for different experimental arrangements. It is the distance from the surface of the IRE at which the amplitude of electric field amplitude falls to 37% (1/e) of its value at the surface:
C, is given by n1/n2. The electric field of the radiation penetrates a short distance into the optically rare medium. The intensity of this electric field, which is known as the evanescent wave, is confined within the vicinity of the surface of the denser medium. Its intensity decreases exponentially with distance, normal to the surface, into the optically rare medium. It can, therefore, be envisaged as penetrating the surface layer of the rarer medium. The depth of penetration, dp, is a convenient comparative term for different experimental arrangements. It is the distance from the surface of the IRE at which the amplitude of electric field amplitude falls to 37% (1/e) of its value at the surface:
n12 = n2/n1
Note that, to achieve total internal reflection, 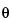 must be greater than
must be greater than 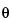 C, so that n2 is usually greater than 2.3. Some of the more commonly used IRE materials are the following: zinc selenide (n2 ~ 2.4), Type IIa diamond (n2 ~ 2.4), silicon (n2 ~ 3.4), and germanium (n2 ~ 4.0). Furthermore, dp decreases with increasing angle of incidence and increases with increasing wavelength (decreasing wavenumber). As a consequence of the increase in dp with increasing wavelength, the band intensities within an ATR spectrum appear—by comparison with a conventional transmission spectrum—to be relatively increasingly enhanced with decreasing wavenumber.
C, so that n2 is usually greater than 2.3. Some of the more commonly used IRE materials are the following: zinc selenide (n2 ~ 2.4), Type IIa diamond (n2 ~ 2.4), silicon (n2 ~ 3.4), and germanium (n2 ~ 4.0). Furthermore, dp decreases with increasing angle of incidence and increases with increasing wavelength (decreasing wavenumber). As a consequence of the increase in dp with increasing wavelength, the band intensities within an ATR spectrum appear—by comparison with a conventional transmission spectrum—to be relatively increasingly enhanced with decreasing wavenumber.
Furthermore, the refractive index of all molecules is not constant and varies across absorption bands (anomalous dispersion). For strong absorption bands, n1 can vary between approximately 1 and 2. This effect causes a shift in the measured wavenumber of a band with respect to transmission spectra. Particularly when equipment is operating at high angles of incidence or with an IRE with a low refractive index such as zinc selenide or diamond, strong bands may also be accompanied by the appearance of an underlying first-derivative-like shape (see above for a discussion of the Christiansen effect in KBr disks).
Many different geometrical shapes and sizes are used for IREs. A trapezoidal IRE is commonly incorporated into so-called horizontal ATR (H-ATR) units. Hemispherical IREs are the core of some micro-sampling ATR accessories. Rod or rod-like multiple internal reflection IREs are often used for on-line monitoring of liquid processes.
Multiple internal reflection (MIR) elements allow the internally reflected radiation in the IRE to interact several or many times with the surface layer of the sample with which it is in contact, thereby increasing the intensity (effective path length) of the recorded sample spectrum. Still, the spectrum recorded is characteristic only of the depth probed by a single reflection. The number of internal reflections depends on the length and thickness of the IRE. MIR elements may be several centimeters in length. A typical configuration for a vertically mounted 45 angle of incidence IRE may allow 25 internal reflections. Today the most commonly used MIR systems in the pharmaceutical laboratory are those in which the IRE is mounted horizontally. These are often referred to as H-ATR accessories. MIR systems based on cylindrical rods with cone-shaped ends or similar geometries are often incorporated as liquid-sampling devices in flow-through solution cells or are used for on-line process monitoring. The trapezoidal-shaped MIR elements incorporated into H-ATR accessories enable or facilitate study of a wide range of sample forms, including liquids, solutions, dispersions, creams, pastes, waxes, semi-solids and soft powders, continuous flat surface solids, solutions, films cast from solution, and many more. Micro– and macro–H-ATR accessories are commercially available with 1, 3, 9, or more sample-interaction reflections.
angle of incidence IRE may allow 25 internal reflections. Today the most commonly used MIR systems in the pharmaceutical laboratory are those in which the IRE is mounted horizontally. These are often referred to as H-ATR accessories. MIR systems based on cylindrical rods with cone-shaped ends or similar geometries are often incorporated as liquid-sampling devices in flow-through solution cells or are used for on-line process monitoring. The trapezoidal-shaped MIR elements incorporated into H-ATR accessories enable or facilitate study of a wide range of sample forms, including liquids, solutions, dispersions, creams, pastes, waxes, semi-solids and soft powders, continuous flat surface solids, solutions, films cast from solution, and many more. Micro– and macro–H-ATR accessories are commercially available with 1, 3, 9, or more sample-interaction reflections.
Single-reflection, simple prism, and novel design IREs are also used in commercial H-ATR units. They provide an effective and convenient means of analyzing and studying samples in a diverse range of physical forms. In particular, small contact area, single-reflection, fixed angle of incidence H-ATR accessories have become popular no-preparation sampling devices within the pharmaceutical industry because they provide a ready means to record, in a simple, quick manner, a mid-IR spectrum from a limited quantity of almost any condensed-phase material. Hemispherical IREs act as focusing lenses so that the area sampled is generally smaller than that sampled with prismatic IREs. Even though the IRE may be opaque to visible light, many accessories listed above allow some form of viewing capability so that the sample under test can be inspected.
Single-reflection micro-ATR units have the advantage of virtually no requirement for a solid sample to have a uniformly flat surface. The test sample is placed in contact with the IRE sampling area and, if it is a solid, a clamp is used to compress and secure the sample against the IRE. Hemispherical ATR elements of zinc selenide, germanium, and silicon also form the sensing elements for ATR objectives that can be fitted to mid-IR FT-IR microscopes. Several of the accessories are capable of operation at controlled elevated temperatures that permit, for instance, studies relating to thermally induced, solid-state form transformations.
The hardness, scratch resistance, chemical inertness, and mid-IR transparency over a wide wavenumber range make the Type IIa diamond a unique material for ATR measurements. Even though it does have a broad absorption feature between approximately 2400 and 2000 cm 1, for most pharmaceutical applications, this is not prohibitive because this is the region in which only characteristic stretching bands occur for triple and cumulated double bonds. Because of cost, the use of Type IIa diamond as an IRE material is usually restricted to micro-ATR accessories or when the IRE is used as the sensing element in an ATR immersion probe for process monitoring. Diamond has a refractive index that is closely matched to that of zinc selenide, so composite IREs can be constructed. Lower-cost focusing or support optics made from zinc selenide can be optically interfaced with a Type IIa diamond ATR sensing element, thereby minimizing the overall cost of the IRE while still benefiting from the properties of the diamond. On the basis of this technology, 3- and 9-reflection MIR configurations have been designed for both laboratory systems and process probes.
1, for most pharmaceutical applications, this is not prohibitive because this is the region in which only characteristic stretching bands occur for triple and cumulated double bonds. Because of cost, the use of Type IIa diamond as an IRE material is usually restricted to micro-ATR accessories or when the IRE is used as the sensing element in an ATR immersion probe for process monitoring. Diamond has a refractive index that is closely matched to that of zinc selenide, so composite IREs can be constructed. Lower-cost focusing or support optics made from zinc selenide can be optically interfaced with a Type IIa diamond ATR sensing element, thereby minimizing the overall cost of the IRE while still benefiting from the properties of the diamond. On the basis of this technology, 3- and 9-reflection MIR configurations have been designed for both laboratory systems and process probes.
External Reflection Spectroscopy
Several types of external reflection IR spectra can be measured. Among them are Fresnel reflection, transflection, reflection–absorption spectroscopy, and photoacoustic spectroscopy, but with the exception of diffuse reflection they are not widely used in pharmaceutical applications.
Diffuse Reflection
Spectra recorded from powders or fairly fine granular samples are known as diffuse reflection (DR) spectra. Most of the spectrum originates from radiation that has penetrated through the surface of the sample and has been transmitted through multiple particles. A relatively small fraction of the DR spectrum originates from radiation that has been reflected from the front surface of the samples and therefore has the shape of a Fresnel reflection spectrum. Because the shapes of bands in mid-IR Fresnel reflection spectra are asymmetrical, the fraction of Fresnel reflection that contributes to a DR spectrum should be reduced to be as small as possible. This may be achieved in a number of ways, the most important and commonly used of which is to dilute the sample by mixing it with 90%–99% of a nonabsorbing diluent such as finely powdered potassium bromide or potassium chloride. The sample dilution has the added benefit of reducing absorption band intensities to an appropriate level.
DR spectra largely result from photons that have been transmitted through tens to hundreds of particles and, therefore, have an appearance similar to that of transmission spectra. However, DR spectra do not obey Beer’s Law. Instead, the DR spectrum measured at infinite depth, R (i.e., band intensities do not change if the thickness of sample is increased), is converted by the Kubelka–Munk function:
(i.e., band intensities do not change if the thickness of sample is increased), is converted by the Kubelka–Munk function:
The function f (R ) is equal to the ratio of the absorption coefficient to the scattering coefficient of the sample. DR is usually calculated by taking the ratio of the single-beam spectrum of the diluted sample to the single-beam spectrum of the neat diluent. Ideally, the sample is ground to the point that the average particle diameter is <5 µm. The sample–diluent mixture is thick enough that any increase in its thickness does not lead to a change in the spectrum. Samples measured in this way are said to be measured at infinite depth, and the reflectance is given the symbol, R
) is equal to the ratio of the absorption coefficient to the scattering coefficient of the sample. DR is usually calculated by taking the ratio of the single-beam spectrum of the diluted sample to the single-beam spectrum of the neat diluent. Ideally, the sample is ground to the point that the average particle diameter is <5 µm. The sample–diluent mixture is thick enough that any increase in its thickness does not lead to a change in the spectrum. Samples measured in this way are said to be measured at infinite depth, and the reflectance is given the symbol, R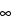 . For mid-IR DR spectrometry, the infinite depth criterion is usually obeyed when the thickness is at least 100 µm, but to be conservative the depth of most sampling cups for mid-IR DR spectrometry is at least 1 mm.
. For mid-IR DR spectrometry, the infinite depth criterion is usually obeyed when the thickness is at least 100 µm, but to be conservative the depth of most sampling cups for mid-IR DR spectrometry is at least 1 mm.
A common way of preparing samples for DR spectrometry is to overfill the cup and to level the sample with a spatula or razor blade. However, this way of preparing the sample can lead to a difference between the scattering coefficient near the surface and in the bulk of the sample. Because the intensity of DR spectra depends on the scattering coefficient, a better sample preparation procedure is to slightly overfill the cup and to tap the base of the cup on a bench until the top surface is level with the rim of the cup.
Several types of accessories are used for the measurement of DR spectra. On-axis, or bright-field, DR accessories are similar to very efficient specular reflection accessories. They are the most efficient devices for the measurement of DR spectra but generally give the least rejection of Fresnel reflection. Off-axis, or dark-field, DR accessories are less efficient than on-axis devices but reject specular reflection more efficiently. Compound parabolic concentrators also have been adapted for DR measurements and appear to be intermediate in efficiency and Fresnel reflection between on-axis and off-axis accessories. Finally, integrating spheres have been used for DR spectrometry. These devices are the most accurate photometrically but are the least efficient.
MICROSPECTROSCOPY AND IMAGING
Transmission Microscopy
In any microscope, the area of a sample under study is defined by one or more remote masking apertures mounted at focused image planes conjugate to the sample focus plane. If the sample is mounted on a computer-controlled x–y stage, then successive neighboring or specified regions can be measured sequentially. These single-point spectra can be used to generate absorbance-intensity contour maps or false-color images that highlight differences or inhomogeneities across the area mapped. Such maps can also be generated by moving the sample manually, but this procedure can be very time consuming. The linear dimension of the smallest sample that can be studied by mid-IR FT-IR microscopy is approximately equal to the wavelength, i.e., approximately 10 µm. In practice, one can usually record a single-point spectrum of acceptable signal-to-noise ratio (SNR) and spectral resolution within a reasonable time-scale from a masked sample area of 10-µm diameter in about 1 min. For mapping over a relatively large sample area, where hundreds or even thousands of spectra are needed, a much more time-efficient process is to use an FT-IR microscope equipped with an array detector. Variable-temperature studies with thermomicroscopy observations and variable-temperature studies undertaken on an FT-IR microscope can be particularly useful for studying solid-state form and thermally induced transitions in situ.
As with macroscopic sampling, micro-samples presented for transmission FT-IR microscopy measurements should be flat. For a full mid-IR fingerprint spectrum, they should be of an appropriate thickness which, in the case of many APIs, may require a sample of approximately 10-µm thickness or less. Compression cells are particularly useful for thinning a sample. A thinned sample can be examined under compression or (less preferably) with the top window of the cell removed and the thinned sample examined while supported on the bottom window. A good practical means of providing an appropriate sample from which to record a single-beam background spectrum is to mount a small particle of potassium bromide alongside the sample and thin both by compression under the same conditions. The single-beam spectra of the sample and reference materials then can be measured under the same conditions simply by moving the compression cell using the x–y stage of the microscope.
Many continuous solids such as polymers used in packing or fibers found as contaminants can be prepared to an appropriate thickness and examined in a compression cell by FT-IR microscopy. Rolling with a tool specifically designed for this purpose can also sometimes decrease the thickness of soft samples. Laminated samples can be sectioned using a microtome and can be examined either free-standing or supported on an IR-transparent window. Analysts commonly use a microtome to get a cross-section from a multi-layer polymer laminate film so that its layer structure can be analyzed by FT-IR microscopy.
Reflection Microscopy
All major types of reflection spectroscopy discussed above can be implemented on microscopes. Most microscopes have optical configurations that enable them to be switched from transmission to reflection measurements if the operator switches a simple flip mirror. The angle of incidence for these measurements is usually between 30 and 45
and 45 so that both Fresnel reflection and transflection measurements can be carried out easily. In contrast, special microscope objectives are required for ATR or reflection–absorption spectrophotometry.
so that both Fresnel reflection and transflection measurements can be carried out easily. In contrast, special microscope objectives are required for ATR or reflection–absorption spectrophotometry.
For ATR microspectroscopy, the sample under investigation is supported on the microscope stage, and the ATR objective is lowered until the IRE is in optical contact with the uppermost surface of the sample. Reproducible contact pressure can be achieved by the use of a pressure gauge. A disadvantage of using a germanium IRE is that, unlike zinc selenide, it does not transmit visible light, which precludes in situ visual inspection of the sample. ATR mapping using a computer-controlled mapping stage in a manner analogous to that of transmission mapping is also possible, but this is usually restricted to soft materials.
Hyperspectral Imaging
The term hyperspectral imaging came into use to describe the process whereby a focal plane array (FPA) detector is used to record simultaneously an array of spectra from a stationary sample. Each pixel of the array records a spectrum of a different region of the sample. This approach is a much more time-efficient process than single-point mapping if many spectra are measured from a large sample area. Typical array sizes for mid-IR applications are 256 × 256, 128 × 128, and 64 × 64. The individual mercury cadmium telluride (MCT) detector elements (pixels) of most FPAs are 6.25 µm × 6.25 µm. The detectors that are incorporated in the FPA are photovoltaic (PV) detectors, unlike the single-element photoconductive (PC) MCT detectors that are used for many FT-IR measurements (see below). Whereas PC MCT detectors operate at least to 750 cm 1, PV detectors that are installed in FPAs typically have a low wavenumber cut-off in the region of approximately 900 cm
1, PV detectors that are installed in FPAs typically have a low wavenumber cut-off in the region of approximately 900 cm 1. Advances in digital electronics and FPA design enable 64 × 64 MCT FPAs to be used with an FT-IR spectrophotometer operating in the more conventional continuous-scan mode. The three-dimensional array of data sets, two spatial and one spectral, recorded in such an imaging measurement has become known as a hypercube or data cube.
1. Advances in digital electronics and FPA design enable 64 × 64 MCT FPAs to be used with an FT-IR spectrophotometer operating in the more conventional continuous-scan mode. The three-dimensional array of data sets, two spatial and one spectral, recorded in such an imaging measurement has become known as a hypercube or data cube.
An alternative way of hyperspectral imaging that is now commercially available is a hybrid approach in which a linear array of small PC MCT detectors is used in combination with a computer-controlled mapping stage. Adjacent regions are rapidly repositioned under the array detector until the full spatial region of interest has been covered. The full field image is built up as a mosaic of the individual area images recorded. In one system, which incorporates a 16-detector linear array, the lower wavenumber cut-off is approximately 700 cm 1.
1.
INSTRUMENTATION
The majority of mid-IR spectra are measured with an FT-IR spectrophotometer. These instruments generally incorporate an incandescent silicon carbide (Globar®-type) source. The radiation emitted by the source is collimated and passed into a continuous scanning two-beam interferometer. The rate of change of optical path difference in the interferometer (usually known as the optical velocity) is typically on the order of 0.2–5.0 cm·s 1. The actual value depends on the detector and the analog-to-digital converter (ADC). The beam emerging from the interferometer is then focused at the center of the sample compartment of the spectrophotometer by an off-axis paraboloidal mirror. After being transmitted through, or reflected from, the sample, the beam is focused onto the detector. This signal is called an interferogram. The interferogram is a record of the variation of the AC component of the energy incident on the detector as a function of the optical path difference (retardation) of the interferometer. The Fourier transform of the interferogram is the single-beam spectrum.
1. The actual value depends on the detector and the analog-to-digital converter (ADC). The beam emerging from the interferometer is then focused at the center of the sample compartment of the spectrophotometer by an off-axis paraboloidal mirror. After being transmitted through, or reflected from, the sample, the beam is focused onto the detector. This signal is called an interferogram. The interferogram is a record of the variation of the AC component of the energy incident on the detector as a function of the optical path difference (retardation) of the interferometer. The Fourier transform of the interferogram is the single-beam spectrum.
The measurement usually involves passing a laser beam through the center of the interferometer, with the resulting sinusoidal interferogram measured by a detector of visible radiation at the same time the IR interferogram is measured by the IR detector. The usual laser used for this purpose is a helium–neon (HeNe) laser with a wavelength of 632.8 nm (15,802 cm 1). This laser interferogram allows the exact position of the moving optical element in the interferometer to be determined. In FT-IR spectrophotometers equipped with an externally triggerable ADC, the IR signal is digitized at each wavelength of the laser interferogram, typically at the zero crossings. If the IR interferogram is sampled once per wavelength of a HeNe laser, the spectral range is restricted to 0–7901 cm
1). This laser interferogram allows the exact position of the moving optical element in the interferometer to be determined. In FT-IR spectrophotometers equipped with an externally triggerable ADC, the IR signal is digitized at each wavelength of the laser interferogram, typically at the zero crossings. If the IR interferogram is sampled once per wavelength of a HeNe laser, the spectral range is restricted to 0–7901 cm 1, i.e., one-half the wavenumber of the laser. In some contemporary FT-IR spectrophotometers, a sigma-delta ADC is used. These ADCs sample the interferogram at constant time intervals rather than at constant intervals of optical path difference, but they have a greater dynamic range than do externally triggered ADCs. In the latter case, the instrument’s software calculates what the value of the interferogram would have been at the laser zero crossings.
1, i.e., one-half the wavenumber of the laser. In some contemporary FT-IR spectrophotometers, a sigma-delta ADC is used. These ADCs sample the interferogram at constant time intervals rather than at constant intervals of optical path difference, but they have a greater dynamic range than do externally triggered ADCs. In the latter case, the instrument’s software calculates what the value of the interferogram would have been at the laser zero crossings.
In obtaining the spectrum of a sample, the single-beam spectrum of the sample and an appropriate reference are measured, and the ratio of these two single-beam spectra is calculated. If the spectrum is obtained in the transmission mode, the ratio is known as the transmittance spectrum, T( ). For many measurements, the negative logarithm of T(
). For many measurements, the negative logarithm of T( ) is calculated to give the absorbance, A(
) is calculated to give the absorbance, A( ). If the sample is interrogated in the reflection mode, the ratio is the reflectance spectrum, R(
). If the sample is interrogated in the reflection mode, the ratio is the reflectance spectrum, R( ). For ATR, transflection and reflection–absorption measurements, R(
). For ATR, transflection and reflection–absorption measurements, R( ), are usually converted to absorbance in the same way as for transmission spectroscopy. Conversion to A(
), are usually converted to absorbance in the same way as for transmission spectroscopy. Conversion to A( ) is essential for quantitative measurements. For Fresnel reflection measurements, R(
) is essential for quantitative measurements. For Fresnel reflection measurements, R( ) is often subjected to a Kramers–Kronig transformation to yield the absorption index spectra, k(
) is often subjected to a Kramers–Kronig transformation to yield the absorption index spectra, k( ), and the refractive index spectra, n(
), and the refractive index spectra, n( ). For mid-IR diffuse reflection measurements, R(
). For mid-IR diffuse reflection measurements, R( ) is usually converted by the Kubelka–Munk function (see Equation 4).
) is usually converted by the Kubelka–Munk function (see Equation 4).
The standard detector used in FT-IR spectrophotometers is a room-temperature pyroelectric bolometer, most commonly deuterated triglycine sulfate (DTGS) or deuterated l-alanine-doped triglycine sulfate. These detectors respond to IR radiation of all wavelengths, and their low wavenumber cut-off is determined by the window behind which they are mounted. The window is typically selected to match the material on which the beamsplitter is deposited and is usually potassium bromide, so that the range is restricted to 400 cm 1.
1.
When the sensitivity of pyroelectric bolometers is inadequate, e.g., for measurements made through a microscope or with a gas chromatography interface, the more sensitive MCT detector is used. MCT detectors are generally operated in the photoconductive mode and are usually cooled to 77 K with liquid nitrogen (LN2). Thermoelectrically cooled MCT detectors are available, but their low wavenumber cut-off is usually well above 1000 cm 1, and they are not as sensitive as LN2-cooled MCT detectors. Liquid nitrogen-cooled indium antimonide (InSb) detectors can also be used. These detectors are more sensitive than MCT but have a cut-off at 1800 cm
1, and they are not as sensitive as LN2-cooled MCT detectors. Liquid nitrogen-cooled indium antimonide (InSb) detectors can also be used. These detectors are more sensitive than MCT but have a cut-off at 1800 cm 1.
1.
Two problems with MCT detectors should be noted. First, because they are more sensitive than DTGS detectors, they should be used only when the sample or sampling accessory attenuates the beam by at least a factor of 10. Otherwise, the ADC will be overloaded and the photometric accuracy of the spectrophotometer will be seriously affected. If the instrument is equipped only with an MCT detector (a rare circumstance), a neutral-density filter can be mounted in the spectrophotometer beam to reduce the energy of the beam at the detector to an appropriate level. Second, even when the ADC is not saturated, the response of MCT detectors is often nonlinear at high signal levels near the centerburst of the interferogram. The effect of this nonlinearity is to cause the baseline of the calculated single-beam spectrum to be displaced from zero. This effect can be seen readily when one plots the single-beam spectrum between 0 and 4000 cm 1. If the average energy in the single-beam spectrum between, for instance, 400 and 300 cm
1. If the average energy in the single-beam spectrum between, for instance, 400 and 300 cm 1 is above or below zero, the detector is responding in a nonlinear manner in the region of the centerburst, and the photometric accuracy of the measurement is degraded concomitantly. Some vendors supply software to correct for this effect, but in general the signal should be reduced by inserting a neutral-density filter, not an aperture stop, in the beam.
1 is above or below zero, the detector is responding in a nonlinear manner in the region of the centerburst, and the photometric accuracy of the measurement is degraded concomitantly. Some vendors supply software to correct for this effect, but in general the signal should be reduced by inserting a neutral-density filter, not an aperture stop, in the beam.
The main factor that affects the resolution of an FT-IR spectrophotometer is the maximum optical path difference of the interferogram. The nominal resolution, D , is the reciprocal of the maximum optical path difference.
, is the reciprocal of the maximum optical path difference.
The divergence angle of the beam passing through the interferometer may also degrade the resolution. The effective collimation of the beam that passes through the interferometer is determined by the limiting aperture of the optical system. In instruments designed for high resolution (D > 0.5 cm
> 0.5 cm 1), an adjustable aperture that serves the same purpose as the entrance aperture of a monochromator is installed at a focus between the source and the interferometer. As the desired resolution is increased (i.e., D
1), an adjustable aperture that serves the same purpose as the entrance aperture of a monochromator is installed at a focus between the source and the interferometer. As the desired resolution is increased (i.e., D is made numerically smaller), the diameter of this aperture, which is known as the Jacquinot stop or J-stop, is decreased, making the divergence angle of the beam in the interferometer smaller;
is made numerically smaller), the diameter of this aperture, which is known as the Jacquinot stop or J-stop, is decreased, making the divergence angle of the beam in the interferometer smaller;
 1), the detector serves the purpose of the Jacquinot stop.
1), the detector serves the purpose of the Jacquinot stop.
If the true full-width at half-height of the bands or lines in the spectrum is less than D , side lobes are seen on each narrow spectral feature. These side lobes can be eliminated by apodization, i.e., multiplying the interferogram by a function that is equal to 1 at the centerburst and decays monotonically with optical path difference. Besides reducing the amplitude of the side lobes, apodization also has the effect of degrading the resolution (broadening the bands). When an interferogram is not weighted, it is often (incorrectly) said to be apodized with a boxcar apodization function, although a better term would be a boxcar truncation function. The selection of apodization function should be made on the basis of the purpose of the experiment, but it is usually limited to a few choices.
, side lobes are seen on each narrow spectral feature. These side lobes can be eliminated by apodization, i.e., multiplying the interferogram by a function that is equal to 1 at the centerburst and decays monotonically with optical path difference. Besides reducing the amplitude of the side lobes, apodization also has the effect of degrading the resolution (broadening the bands). When an interferogram is not weighted, it is often (incorrectly) said to be apodized with a boxcar apodization function, although a better term would be a boxcar truncation function. The selection of apodization function should be made on the basis of the purpose of the experiment, but it is usually limited to a few choices.
The Norton–Beer weak, medium, and strong apodization functions give the optimum combination of side-lobe amplitude for a loss in resolution of 20%, 40%, and 60%, respectively. The Norton–Beer medium apodization function is generally appropriate for many measurements of condensed-phase samples. The Happ–Genzel apodization function is also a nearly optimal function that degrades the resolution by about 50% (i.e., it is midway between the Norton–Beer medium and strong functions). A commonly available function is the triangular apodization function. This function has the effect of causing significant deviations from Beer's Law and is not recommended.
Wavenumber Accuracy
The main factors that affect wavenumber accuracy are the alignment of the laser and IR beams in the interferometer and the divergence of the beam passing through the interferometer as observed with the IR detector. One might think that because the wavenumber of the laser beam is known very accurately, the wavenumber scale of a spectrum measured on an FT-IR spectrophotometer should be known to equal accuracy. However, the laser beam is highly collimated, whereas the beam from the IR source is not (a collimated beam can be obtained only from a point source). Because both the source and detector have a finite size, equal accuracy (of the wavenumber of the laser beam and the wavenumber scale of the spectrum measured) is never the case. As the diameter of the Jacquinot stop is decreased, a small wavenumber shift (always less than 0.25 D ) will be observed. When the diameter of a sample is less than the diameter of the beam focus in the sample compartment or the sampling accessory, the effect is also to vignette the beam (i.e., to stop the beam down), and a small wavelength shift will be observed.
) will be observed. When the diameter of a sample is less than the diameter of the beam focus in the sample compartment or the sampling accessory, the effect is also to vignette the beam (i.e., to stop the beam down), and a small wavelength shift will be observed.
Photometric Accuracy
The main factor that affects the photometric accuracy of FT-IR spectrophotometers is the linearity of the detector response. As noted above, the response of pyroelectric bolometers usually varies linearly with the energy on the detector, but this is not the case for MCT detectors. With MCT detectors, the best way of detecting photometric error is measurement of the nonphysical energy in the single-beam spectrum below the detector cut-off.
Sensitivity
The sensitivity of the instrument can be determined by measuring two single-beam spectra under exactly the same conditions and calculating their ratio to produce what is commonly known as a 100% line. The noise level in different spectral regions can be estimated either as the peak-to-peak noise, i.e., the difference between the maximum and minimum values of the percent transmission in the selected spectral region(s), or the root-mean-square (RMS) noise, i.e., the standard deviation of the spectrum in that region. The RMS noise level is the preferred metric because this calculation involves all the data in the selected region rather than just the two most deviant points. An example of suitable measurement conditions to test the sensitivity of an FT-IR spectrophotometer equipped with a DTGS detector are 16 co-added scans, a resolution of 2 cm 1, and Norton–Beer medium apodization. The most commonly used spectral region is 2200–2000 cm
1, and Norton–Beer medium apodization. The most commonly used spectral region is 2200–2000 cm 1 because (a) this is where the performance of most mid-IR spectrophotometers is highest, and (b) no common atmospheric interferent such as water or carbon dioxide absorbs strongly in this region. However, other regions should be tested close to the ends of the spectrum, such as 650–450 cm
1 because (a) this is where the performance of most mid-IR spectrophotometers is highest, and (b) no common atmospheric interferent such as water or carbon dioxide absorbs strongly in this region. However, other regions should be tested close to the ends of the spectrum, such as 650–450 cm 1 and 4000–3800 cm
1 and 4000–3800 cm 1. The SNR of the spectrophotometer operating with certain parameters in a given spectral region is estimated as 100/(RMS noise level in percent transmission).
1. The SNR of the spectrophotometer operating with certain parameters in a given spectral region is estimated as 100/(RMS noise level in percent transmission).
Beer's Law Linearity
For quantitative measurements, the spectrum is measured at a resolution that is at least twice as narrow as the narrowest band in the spectrum. The use of either the Norton–Beer medium or Happ–Genzel apodization function is recommended. For optimal photometric accuracy, the maximum absorbance of the analytical bands is no greater than 1.0 absorbance unit. Higher absorbance values can be tolerated with certain combinations of resolution and apodization functions. The effect of band width and peak absorbance on Beer’s Law linearity for strong bands depends on the resolution and apodization function, and should be validated on a case-by-case basis. USP38
USP38
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Margareth R.C. Marques, Ph.D.
Senior Scientific Liaison (301) 816-8106 |
(GCCA2010) General Chapters - Chemical Analysis |
USP38–NF33 Page 1724
Pharmacopeial Forum: Volume No. 40(1)