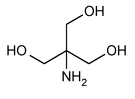
Tromethamine
(troe meth' a meen).
1,3-Propanediol, 2-amino-2-(hydroxymethyl)-.
2-Amino-2-(hydroxymethyl)-1,3-propanediol
» Tromethamine contains not less than 99.0 percent and not more than 101.0 percent of C4H11NO3, calculated on the dried basis.
Packaging and storage—Preserve in tight containers.
Identification—
B: To 4.5 mL of a saturated solution of salicylaldehyde add 0.5 mL of glacial acetic acid, and mix. Add 4.0 mL of a solution of Tromethamine (1 in 5), and mix: a yellow color is produced.
C: To 0.5 mL of a 4 in 10 solution of ceric ammonium nitrate in 2 N nitric acid add 3 mL of water and 0.5 mL of a solution of Tromethamine (1 in 5), and mix: the color changes from light yellow to orange.
Melting range  741
741 : between 168
: between 168 and 172
and 172 .
.
pH  791
791 : between 10.0 and 11.5, in a solution (1 in 20).
: between 10.0 and 11.5, in a solution (1 in 20).
Loss on drying  731
731 —Dry it at 105
—Dry it at 105 for 3 hours: it loses not more than 1.0% of its weight.
for 3 hours: it loses not more than 1.0% of its weight.
Residue on ignition  281
281 : not more than 0.1%.
: not more than 0.1%.
Heavy metals, Method II  231
231 : 0.001%.
: 0.001%.
Assay—Dissolve about 250 mg of Tromethamine, accurately weighed, in 100 mL of water, add bromocresol purple TS, and titrate with 0.1 N hydrochloric acid VS to a yellow endpoint. Each mL of 0.1 N hydrochloric acid is equivalent to 12.11 mg of C4H11NO3.
Auxiliary Information—Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Feiwen Mao, M.S. Senior Scientific Liaison 1-301-816-8320 | (SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4966
Pharmacopeial Forum: Volume No. 35(2) Page 316Chromatographic Column—
Chromatographic columns text is not derived from, and not part of, USP 35 or NF 30.