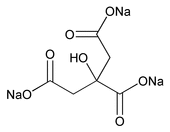
Sodium Citrate
C6H5Na3O7258.07
1,2,3-Propanetricarboxylic acid, 2-hydroxy-, trisodium salt;
Trisodium citrate (anhydrous)

 [68-04-2].
[68-04-2].
C6H5Na3O7·2H2O294.10
Trisodium citrate dihydrate

 [6132-04-3].
[6132-04-3].
(soe' dee um si' trate).
C6H5Na3O7258.07
1,2,3-Propanetricarboxylic acid, 2-hydroxy-, trisodium salt;
Trisodium citrate (anhydrous)
C6H5Na3O7·2H2O294.10
Trisodium citrate dihydrate
DEFINITION
Sodium Citrate is anhydrous or contains two molecules of water of hydration. It contains NLT 99.0% and NMT 100.5% of C6H5Na3O7, calculated on the anhydrous basis.
IDENTIFICATION
• A. Identification Tests—General, Sodium  191
191
Sample solution: 50 mg/mL
Acceptance criteria: Meets the requirements
• B. Identification Tests—General, Citrate  191
191
Sample solution: 50 mg/mL
Acceptance criteria: Meets the requirements
• C. Upon ignition, it yields an alkaline residue that effervesces when treated with 3 N hydrochloric acid.
ASSAY
• Procedure
Sample: Add 100 mL of glacial acetic acid to 350 mg of Sodium Citrate (previously dried at 180 for 18 h) in a 250-mL beaker. Stir until completely dissolved.
for 18 h) in a 250-mL beaker. Stir until completely dissolved.
Analysis: Titrate with 0.1 N perchloric acid VS, determining the endpoint potentiometrically. Perform a blank determination, and make any necessary correction (see Titrimetry  541
541 ). Each mL of 0.1 N perchloric acid is equivalent to 8.602 mg of C6H5Na3O7.
). Each mL of 0.1 N perchloric acid is equivalent to 8.602 mg of C6H5Na3O7.
Acceptance criteria: 99.0%–100.5% on the anhydrous basis
IMPURITIES
• Heavy Metals  231
231
[Note—Use 50-mL color comparison tubes for preparing the Standard preparation, Test preparation, and Monitor preparation. ]
Standard preparation: 1.0 mL of Standard Lead Solution and 11 mL of water
Test stock preparation: 88 mg/mL of anhydrous sodium citrate in water
Test preparation: 12 mL of the Test stock preparation
Monitor preparation: 11 mL of the Test stock preparation and 1.0 mL of Standard Lead Solution
Analysis: Proceed as directed in the chapter for Procedure, omitting the dilution to 50 mL.
Acceptance criteria: NMT 10 ppm
• Tartrate
Analysis: To a solution of 1 g in 2 mL of water in a test tube add 1 mL of potassium acetate TS and 1 mL of 6 N acetic acid. Rub the wall of the tube with a glass rod.
Acceptance criteria: No crystalline precipitate is formed.
SPECIFIC TESTS
• Alkalinity
Sample solution: 1.0 g in 20 mL of water
Acceptance criteria: The Sample solution is alkaline to litmus paper, but after the addition of 0.20 mL of 0.10 N sulfuric acid, no pink color is produced by 1 drop of phenolphthalein TS.
• Water Determination, Method III  921
921 : Dry a sample at 180
: Dry a sample at 180 for 18 h: the anhydrous form loses NMT 1.0% of its weight; the hydrous form loses 10.0%–13.0% of its weight.
for 18 h: the anhydrous form loses NMT 1.0% of its weight; the hydrous form loses 10.0%–13.0% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage: Preserve in tight containers.
• Labeling: Label it to indicate whether it is anhydrous or hydrous.
Auxiliary Information— Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Sujatha Ramakrishna, Ph.D. Senior Scientific Liaison 1-301-816-8349 | (SM22010) Monographs - Small Molecules 2 |
USP35–NF30 Page 4657