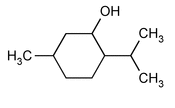
Menthol
(men' thol).
» Menthol is an alcohol obtained from diverse mint oils or prepared synthetically. Menthol may be levorotatory (l-Menthol), from natural or synthetic sources, or racemic (dl-Menthol).
Packaging and storage—Preserve in tight containers, preferably at controlled room temperature.
Labeling—Label it to indicate whether it is levorotatory or racemic.
Identification—When it is triturated with about an equal weight of camphor, of chloral hydrate, or of phenol, the mixture liquefies.
Melting range of l-Menthol  741
741 : between 41
: between 41 and 44
and 44 .
.
Congealing range of dl-Menthol  651
651 —[note—Perform this test preferably in a room having a temperature below 30
—[note—Perform this test preferably in a room having a temperature below 30 and a relative humidity below 50%. ] Place about 10 g of racemic Menthol, previously dried in a desiccator over silica gel for 24 hours, in a dry test tube of from 18- to 20-mm internal diameter, and melt the contents at a temperature of about 40
and a relative humidity below 50%. ] Place about 10 g of racemic Menthol, previously dried in a desiccator over silica gel for 24 hours, in a dry test tube of from 18- to 20-mm internal diameter, and melt the contents at a temperature of about 40 . Suspend the test tube in water having a temperature of 23
. Suspend the test tube in water having a temperature of 23 to 25
to 25 , and stir the contents of the tube continually with a thermometer, keeping the bulb of the thermometer immersed in the liquid. Racemic Menthol congeals at a temperature between 27
, and stir the contents of the tube continually with a thermometer, keeping the bulb of the thermometer immersed in the liquid. Racemic Menthol congeals at a temperature between 27 and 28
and 28 . Shortly after the temperature has stabilized at the congealing point, add a few mg of dried racemic Menthol to the congealed mass, and continue the stirring: after a few minutes the temperature of the mass quickly rises to 30.5
. Shortly after the temperature has stabilized at the congealing point, add a few mg of dried racemic Menthol to the congealed mass, and continue the stirring: after a few minutes the temperature of the mass quickly rises to 30.5 to 32.0
to 32.0 .
.
Specific rotation  781S
781S : between
: between  45
45 and
and  51
51 for l-Menthol; between
for l-Menthol; between  2
2 and +2
and +2 for dl-Menthol.
for dl-Menthol.
Test solution: 100 mg per mL, in alcohol.
Limit of nonvolatile residue—Volatilize 2 g, accurately weighed, in a tared open porcelain dish on a steam bath, and dry the residue at 105 for 1 hour: the residue weighs not more than 1 mg (0.05%).
for 1 hour: the residue weighs not more than 1 mg (0.05%).
Chromatographic purity—
System suitability preparation—Dissolve decanol and Menthol in ether to obtain a solution having concentrations of about 0.05 mg of each per mL.
Test preparation—Dissolve 10 mg of Menthol in 50 mL of ether, and mix. Dilute 25 mL of this solution with ether to 100 mL, and mix.
Chromatographic system—The gas chromatograph is equipped with a flame-ionization detector and contains a 1.8-m × 2-mm column packed with 10% phase G16 on support S1AB. The column is maintained at about 170 , the injection port is maintained at about 260
, the injection port is maintained at about 260 , and the detector block is maintained at about 240
, and the detector block is maintained at about 240 . Dry helium is used as the carrier gas at a flow rate of about 50 mL per minute. Chromatograph the System suitability preparation, and record the peak responses as directed under Procedure: the retention time of menthol is about 0.7 relative to decanol. In a suitable chromatogram, the resolution, R, of the 2 peaks is not less than 2.5, and the relative standard deviation of the ratio of the peak response obtained with menthol to that obtained with decanol is not more than 2%.
. Dry helium is used as the carrier gas at a flow rate of about 50 mL per minute. Chromatograph the System suitability preparation, and record the peak responses as directed under Procedure: the retention time of menthol is about 0.7 relative to decanol. In a suitable chromatogram, the resolution, R, of the 2 peaks is not less than 2.5, and the relative standard deviation of the ratio of the peak response obtained with menthol to that obtained with decanol is not more than 2%.
Procedure—Inject about 2 µL of the Test preparation into the gas chromatograph, and measure the peak responses. The peak response due to menthol is not less than 97% of the sum of all the peak responses, excluding any due to the ether.
Readily oxidizable substances in dl-Menthol—Place 500 mg of dl-Menthol in a clean, dry test tube, add 10 mL of a solution of potassium permanganate, prepared by diluting 3 mL of 0.1 N potassium permanganate with water to 100 mL, and place the test tube in a beaker of water at a temperature between 45 and 50
and 50 . Remove the tube from the bath at intervals of 30 seconds, and mix quickly by shaking: the purple color of potassium permanganate is still apparent after 5 minutes.
. Remove the tube from the bath at intervals of 30 seconds, and mix quickly by shaking: the purple color of potassium permanganate is still apparent after 5 minutes.
Auxiliary Information—Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Maged H. Sharaf, Ph.D. Principal Scientific Liaison 1-301-816-8318 | (DS2010) Monographs - Dietary Supplements |
USP35–NF30 Page 3800
Chromatographic Column—
Chromatographic columns text is not derived from, and not part of, USP 35 or NF 30.