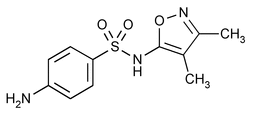
Sulfisoxazole
(sul'' fi sox' a zole).
Benzenesulfonamide, 4-amino-N-(3,4-dimethyl-5-isoxazolyl)-.
N1-(3,4-Dimethyl-5-isoxazolyl)sulfanilamide
» Sulfisoxazole contains not less than 99.0 percent and not more than 101.0 percent of C11H13N3O3S, calculated on the dried basis.
Packaging and storage—
Preserve in tight, light-resistant containers.
Identification—
B:
The UV absorption spectrum of a 1 in 100,000 solution in pH 7.5 phosphate buffer (see Buffer Solutions in the section Reagents, Indicators, and Solutions), prepared by dissolving about 100 mg of Sulfisoxazole, accurately weighed, in 10 mL of sodium hydroxide solution (1 in 250) and adding sufficient amounts of the buffer solution to make 100 mL, then diluting 10 mL of the resulting solution with the buffer solution to 1000 mL, exhibits maxima and minima at the same wavelengths as that of a similar solution of USP Sulfisoxazole RS, concomitantly measured.
C:
Dissolve about 10 mg in 2 mL of 3 N hydrochloric acid with the aid of heat, cool for 5 minutes in an ice bath, add 3 drops of sodium nitrite solution (1 in 100), and dilute with water to 4 mL: a yellow solution is produced. Add 1 mL of sodium hydroxide solution (1 in 10) containing 10 mg of 2-naphthol: an orange-red precipitate is formed.
Melting range  741
741 :
between 194
:
between 194 and 199
and 199 .
.
Loss on drying  731
731 —
Dry it at 105
—
Dry it at 105 for 2 hours: it loses not more than 0.5% of its weight.
for 2 hours: it loses not more than 0.5% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Selenium  291
291 :
0.003%, a 200-mg test specimen being used.
:
0.003%, a 200-mg test specimen being used.
Heavy metals, Method II  231
231 :
0.002%.
:
0.002%.
Ordinary impurities  466
466 —
—
Test solution:
ethyl acetate.
Standard solution:
ethyl acetate.
Eluant:
a mixture of acetone, cyclohexane, and glacial acetic acid (5:4:1).
Visualization:
1.
Assay—
Place about 800 mg of Sulfisoxazole, accurately weighed, in a 250-mL conical flask, add 50 mL of dimethylformamide, shake thoroughly to dissolve the solid, add 5 drops of a 1 in 100 solution of thymol blue in dimethylformamide, and titrate with 0.1 N lithium methoxide in toluene VS to a blue endpoint, taking precautions against absorption of atmospheric carbon dioxide. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N lithium methoxide is equivalent to 26.73 mg of C11H13N3O3S.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Leonel M. Santos, Ph.D.
Senior Scientific Liaison 1-301-816-8168 |
(SM12010) Monographs - Small Molecules 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4725
Pharmacopeial Forum: Volume No. 30(4) Page 1301