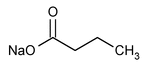
Sodium Butyrate
(soe' dee um bue' ti rate).
» Sodium Butyrate contains not less than 98.0 percent and not more than 101.0 percent of C4H7NaO2, calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers, and store at controlled room temperature.
Labeling—
Label it to indicate that it is intended for use in compounding dosage forms for rectal use only.
Identification—
A:
Infrared Absorption  197K
197K , on the undried specimen.
, on the undried specimen.
B:
A solution (1 in 10) meets the requirements of the tests for Sodium  191
191 .
.
Alkalinity—
Dissolve 2.0 g in 20 mL of water, and add 1 drop of phenolphthalein TS: if a pink color is produced, it is discharged by 0.50 mL of 0.10 N sulfuric acid.
Water, Method I  921
921 :
not more than 1.0%.
:
not more than 1.0%.
Heavy metals, Method II  231
231 :
0.001%.
:
0.001%.
Assay—
Dissolve about 200 mg of Sodium Butyrate, accurately weighed, in 50 mL of glacial acetic acid. Add 1 drop of crystal violet TS, and titrate with 0.1 N perchloric acid to a green endpoint. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 11.01 mg of C4H7NaO2.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Elena Gonikberg, Ph.D.
Principal Scientific Liaison 1-301-816-8251 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4652
Pharmacopeial Forum: Volume No. 28(6) Page 1868