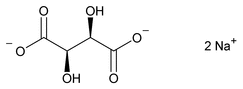Sodium Tartrate
(soe' dee um tar' trate).
DEFINITION
Sodium Tartrate contains NLT 99.0% and NMT 100.5% of sodium tartrate (C4H4Na2O6), calculated on the dried basis.
IDENTIFICATION
• A. Identification Tests—General, Sodium  191
191 :
Meets the requirements
:
Meets the requirements
• B. Identification Tests—General, Tartrate  191
191 :
Meets the requirements
:
Meets the requirements
ASSAY
• Procedure
Sample:
250 mg, previously dried at 150 for 3 h
for 3 h
Titrimetric system
(See Titrimetry  541
541 .)
.)
Mode:
Direct titration
Titrant:
0.1 N Perchloric acid VS (in glacial acetic acid)
Endpoint detection:
Potentiometric
Analysis:
Dissolve the Sample in 150 mL of acetic acid by stirring and heating to near the boiling point. Cool to room temperature. Titrate with 0.1 N perchloric acid VS (in glacial acetic acid) to a potentiometric endpoint. Perform a blank determination, and make any necessary adjustments. Each mL of 0.1 N perchloric acid is equivalent to 9.703 mg of sodium tartrate (C4H4Na2O6).
Acceptance criteria:
99.0%–100.5% on the dried basis
IMPURITIES
• Heavy Metals, Method I  231
231 :
NMT 20 ppm
:
NMT 20 ppm
SPECIFIC TESTS
• pH  791
791 :
7–9 (1 in 10 solution)
:
7–9 (1 in 10 solution)
• Loss on Drying  731
731 :
Dry a sample at 150
:
Dry a sample at 150 for 3 h: it loses 14.0%–17.0% of its weight.
for 3 h: it loses 14.0%–17.0% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in a tight container. No storage requirements specified.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S.
Scientific Liaison 1-301-816-8335 |
(EXC2010) Monographs - Excipients |
USP35–NF30 Page 1962
Pharmacopeial Forum: Volume No. 32(6) Page 1776