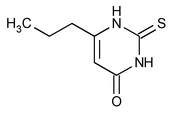
Propylthiouracil
(proe'' pil thye'' oh ure' a sil).
4(1H)-Pyrimidinone, 2,3-dihydro-6-propyl-2-thioxo-.
6-Propyl-2-thiouracil
» Propylthiouracil contains not less than 98.0 percent and not more than 100.5 percent of C7H10N2OS, calculated on the dried basis.
Propylthiouracil contains not less than 98.0 percent and not more than 100.5 percent of C7H10N2OS, calculated on the dried basis.
Packaging and storage—
Preserve in well-closed, light-resistant containers.
Identification, Infrared Absorption  197K
197K .
.
Melting range  741
741 :
between 218
:
between 218 and 221
and 221 .
.
Loss on drying  731
731 —
Dry it at 105
—
Dry it at 105 for 2 hours: it loses not more than 0.5% of its weight.
for 2 hours: it loses not more than 0.5% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Selenium  291
291 :
0.003%, a 200-mg specimen being used.
:
0.003%, a 200-mg specimen being used.
Heavy metals, Method II  231
231 :
0.002%.
:
0.002%.
Ordinary impurities  466
466 —
—
Test solution:
methanol.
Standard solution:
methanol.
Application volume:
10 µL.
Eluant:
a mixture of toluene, ethyl acetate, and formic acid (50:45:5), in a nonequilibrated chamber.
Visualization:
1.
Assay—
Weigh accurately about 300 mg of Propylthiouracil, transfer to a 500-mL conical flask, and add 30 mL of water. Add from a buret about 30 mL of 0.1 N sodium hydroxide VS, heat to boiling, and agitate until solution is complete. Wash down any particles on the wall of the flask with a few mL of water, then add about 50 mL of 0.1 N silver nitrate while mixing, and boil gently for 7 minutes. Cool to room temperature, and continue to titrate with 0.1 N sodium hydroxide VS, determining the endpoint potentiometrically, using a glass-calomel electrode system. Each mL of 0.1 N sodium hydroxide is equivalent to 8.512 mg of C7H10N2OS.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Elena Gonikberg, Ph.D.
Principal Scientific Liaison 1-301-816-8251 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4472