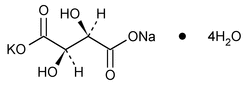Potassium Sodium Tartrate
(poe tas' ee um soe' dee um tar' trate).
Butanedioic acid, 2,3-dihydroxy-, [R-(R*,R*)]-, monopotassium monosodium salt, tetrahydrate.
Monopotassium monosodium tartrate tetrahydrate
Anhydrous 210.16
» Potassium Sodium Tartrate contains not less than 99.0 percent and not more than 102.0 percent of C4H4KNaO6, calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
Identification—
A:
Ignite it: it emits the odor of burning sugar and leaves a residue that is alkaline to litmus and that effervesces with acids.
B:
To 10 mL of a solution (1 in 20) add 10 mL of 6 N acetic acid: a white, crystalline precipitate is formed within 15 minutes.
C:
A solution (1 in 10) responds to the tests for Tartrate  191
191 .
.
Alkalinity—
A solution of 1.0 g in 20 mL of water is alkaline to litmus, but after the addition of 0.20 mL of 0.10 N sulfuric acid no pink color is produced by the addition of 1 drop of phenolphthalein TS.
Water, Method I  921
921 :
between 21.0% and 27.0%.
:
between 21.0% and 27.0%.
Limit of ammonia—
Sodium hypochlorite solution—
Use a commercially available solution that contains between 4.0% and 6.0% of sodium hypochlorite.
Oxidizing solution—
[note—Prepare on the day of use. ] Prepare a mixture of alkaline sodium citrate TS and Sodium hypochlorite solution (4:1).
Diluted sodium nitroferricyanide solution—
Prepare a mixture of water and sodium nitroferricyanide TS (10:1).
Standard solution—
Transfer 300 mg of ammonium chloride, previously dried over silica gel for 4 hours, to a 1-L volumetric flask, and dilute with water to volume. This solution contains 100 µg of ammonia per mL. Dilute this solution quantitatively, and stepwise if necessary, with water to obtain a solution having a concentration of 1.0 µg of ammonia per mL.
Test solution—
Transfer 5.0 g of Potassium Sodium Tartrate to a 100-mL volumetric flask, and dissolve in and dilute with water to volume.
Procedure—
[note—Carefully follow the order of addition stated below. ] Separately transfer 4.0 mL of each of the Standard solution and the Test solution to two color-comparison tubes. To each tube add 0.4 mL of phenol TS, 0.4 mL of Diluted sodium nitroferricyanide solution, and 1.0 mL of Oxidizing solution. Dilute with water to 10 mL, mix, and allow to stand for 1 hour: the color of the Test solution is not darker than the color of the Standard solution (0.002%).
Heavy metals, Method II  231
231 :
0.001%.
:
0.001%.
Assay—
Weigh accurately about 2 g of Potassium Sodium Tartrate in a tared porcelain crucible, and ignite, gently at first, until the salt is thoroughly carbonized, protecting the carbonized salt from the flame at all times. Cool the crucible, place it in a glass beaker, and break up the carbonized mass with a glass rod. Without removing the glass rod or the crucible, add 50 mL of water and 50.0 mL of 0.5 N sulfuric acid VS, cover the beaker, and boil the solution for 30 minutes. Filter, and wash with hot water until the last washing is neutral to litmus. Cool the combined filtrate and washings, add methyl red-methylene blue TS, and titrate the excess acid with 0.5 N sodium hydroxide VS. Perform a blank determination (see Residual Titrations under Titrimetry  541
541 ). Each mL of 0.5 N sulfuric acid is equivalent to 52.54 mg of C4H4KNaO6.
). Each mL of 0.5 N sulfuric acid is equivalent to 52.54 mg of C4H4KNaO6.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Elena Gonikberg, Ph.D.
Principal Scientific Liaison 1-301-816-8251 |
(SM32010) Monographs - Small Molecules 3 |
USP35–NF30 Page 4378
Pharmacopeial Forum: Volume No. 31(3) Page 787