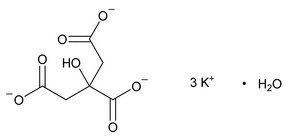
Potassium Citrate
C6H5K3O7·H2O 324.41
C6H5K3O7 306.40
1,2,3-Propanetricarboxylic acid, 2-hydroxy-, tripotassium salt, monohydrate;
Tripotassium citrate monohydrate

 [6100-05-6].
[6100-05-6].
Anhydrous

 [866-84-2].
[866-84-2].
(poe tas' ee um sit' rate).
C6H5K3O7·H2O 324.41
C6H5K3O7 306.40
1,2,3-Propanetricarboxylic acid, 2-hydroxy-, tripotassium salt, monohydrate;
Tripotassium citrate monohydrate
Anhydrous
DEFINITION
Potassium Citrate contains NLT 99.0% and NMT 100.5% of potassium citrate (C6H5K3O7), calculated on the dried basis.
IDENTIFICATION
• A. Identification Tests—General, Potassium  191
191 and Citrate
and Citrate  191
191 :
A 100-mg/mL solution meets the requirements.
:
A 100-mg/mL solution meets the requirements.
ASSAY
• Procedure
Sample:
200 mg of Potassium Citrate
Blank:
25 mL of glacial acetic acid
Titrimetric system
(See Titrimetry  541
541 .)
.)
Mode:
Direct titration
Titrant:
0.1 N perchloric acid VS
Endpoint detection:
Visual
Analysis:
Dissolve the Sample in 25 mL of glacial acetic acid, add 2 drops of crystal violet TS, and titrate with the Titrant to a green endpoint. Perform a Blank determination.
Calculate the percentage of potassium citrate (C6H5K3O7) in the Sample taken:
Result = {[(VS  VB) × N × F]/W} × 100
VB) × N × F]/W} × 100
| VS | = | = Titrant volume consumed by the Sample (mL) |
| VB | = | = Titrant volume consumed by the Blank (mL) |
| N | = | = actual normality of the Titrant (mEq/mL) |
| F | = | = equivalency factor, 102.1 mg/mEq |
| W | = | = Sample weight (mg) |
Acceptance criteria:
99.0%–100.5% on the dried basis
IMPURITIES
• Heavy Metals, Method I  231
231
Test preparation:
Dissolve 2 g in 25 mL of water, and proceed as directed in the chapter, except use glacial acetic acid to adjust the pH.
Acceptance criteria:
NMT 10 ppm
• Tartrate
Sample:
1 g of Potassium Citrate
Analysis:
Dissolve the Sample in 1.5 mL of water in a test tube, add 1 mL of 6 N acetic acid, and scratch the walls of the test tube with a glass rod.
Acceptance criteria:
No crystalline precipitate is formed.
SPECIFIC TESTS
• Alkalinity
Sample solution:
Dissolve 1 g of Potassium Citrate in 20 mL of water.
Analysis:
To the Sample solution add 0.20 mL of 0.10 N sulfuric acid, and add 1 drop of phenolphthalein TS.
Acceptance criteria:
The Sample solution is alkaline to litmus. No pink color is produced after the addition of phenolphthalein TS.
• Loss on Drying  731
731 :
Dry a sample at 180
:
Dry a sample at 180 for 4 h: it loses 3.0%–6.0% of its weight.
for 4 h: it loses 3.0%–6.0% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Huy T. Dinh, M.S.
Scientific Liaison 1-301-816-8594 |
(DS2010) Monographs - Dietary Supplements |
USP35–NF30 Page 4366