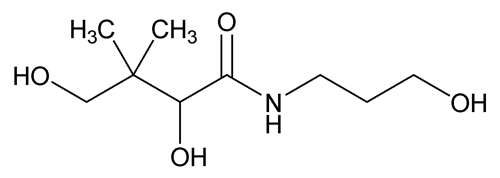
Panthenol
C9H19NO4 205.25
Butanamide, 2,4-dihydroxy-N-(3-hydroxypropyl)-3,3-dimethyl-, (±)-;
(±)-2,4-Dihydroxy-N-(3-hydroxypropyl)-3,3-dimethylbutyramide;
(±)-Pantothenyl alcohol

 [16485-10-2].
[16485-10-2].
(pan' the nol).
C9H19NO4 205.25
Butanamide, 2,4-dihydroxy-N-(3-hydroxypropyl)-3,3-dimethyl-, (±)-;
(±)-2,4-Dihydroxy-N-(3-hydroxypropyl)-3,3-dimethylbutyramide;
(±)-Pantothenyl alcohol
DEFINITION
Panthenol is a racemic mixture of the dextrorotatory and levorotatory isomers of panthenol. It contains NLT 99.0% and NMT 102.0% of racemic panthenol (C9H19NO4), calculated on the dried basis.
IDENTIFICATION
• B.
Sample solution:
100 mg/mL of Panthenol
Analysis:
To 1 mL of the Sample solution add 5 mL of 1 N sodium hydroxide and 1 drop of cupric sulfate TS, and shake vigorously.
Acceptance criteria:
A deep blue color develops.
• C.
Sample solution:
10 mg/mL of Panthenol
Analysis:
To 1 mL of the Sample solution add 1 mL of 1 N hydrochloric acid, and heat on a steam bath for 30 min. Cool, add 100 mg of hydroxylamine hydrochloride, and add 5 mL of 1 N sodium hydroxide. Allow to stand for 5 min, then adjust with 1 N hydrochloric acid to a pH of 2.5–3.0, and add 1 drop of ferric chloride TS.
Acceptance criteria:
A purplish red color develops.
ASSAY
• Procedure
0.1 M potassium biphthalate:
Transfer 20.42 g of potassium biphthalate into a 1000-mL volumetric flask, and add sufficient glacial acetic acid to dissolve. If necessary, warm the mixture on a steam bath to achieve complete solution, observing precautions against absorption of moisture. Cool to room temperature, and dilute with glacial acetic acid to volume.
Sample:
400 mg of Panthenol
Blank:
Proceed as directed in the Analysis, omitting the Sample.
Titrimetric system
(See Titrimetry  541
541 .)
.)
Mode:
Residual titration
Titrant:
0.1 N perchloric acid VS
Back-titrant:
0.1 M potassium biphthalate
Endpoint detection:
Visual
Analysis:
Transfer the Sample to a 300-mL flask fitted to a reflux condenser by means of a standard-taper glass joint, add 50.0 mL of Titrant, and reflux for 5 h. Cool, observing precautions to prevent atmospheric moisture from entering the condenser, and rinse the condenser with glacial acetic acid, collecting the rinsings in the flask. To the flask add 5 drops of crystal violet TS, and titrate with the Back-titrant to a blue-green endpoint. Perform the Blank determination.
Calculate the percentage of panthenol (C9H19NO4) in the Sample taken:
Result = {[(VB  VS) × M × F]/W} × 100
VS) × M × F]/W} × 100
| VB | = | = Back-titrant volume consumed by the Blank (mL) |
| VS | = | = Back-titrant volume consumed by the Sample (mL) |
| M | = | = actual molarity of the Back-titrant (mM/mL) |
| F | = | = equivalency factor, 205.3 mg/M |
| W | = | = Sample weight (mg) |
Acceptance criteria:
99.0%–102.0% on the dried basis
IMPURITIES
• Residue on Ignition  281
281 :
NMT 0.1%
:
NMT 0.1%
• Limit of Aminopropanol
Sample:
10 g of Panthenol
Blank:
25 mL of water
Titrimetric system
(See Titrimetry  541
541 .)
.)
Mode:
Direct titration
Titrant:
0.01 N sulfuric acid VS
Endpoint detection:
Visual
Analysis:
Dissolve the Sample in 25 mL of water, and add bromothymol blue TS. Titrate with the Titrant to a yellow endpoint. Perform the Blank determination.
Calculate the percentage of aminopropanol in the Sample taken:
Result = {[(VS  VB) × N × F]/W} × 100
VB) × N × F]/W} × 100
| VS | = | = Titrant volume consumed by the Sample (mL) |
| VB | = | = Titrant volume consumed by the Blank (mL) |
| N | = | = actual normality of the Titrant (mEq/mL) |
| F | = | = equivalency factor, 75.11 mg/mEq |
| W | = | = Sample weight (mg) |
Acceptance criteria:
NMT 0.10%
SPECIFIC TESTS
• Melting Range, Class I  741
741 :
64.5
:
64.5 –68.5
–68.5
• Optical Rotation, Specific Rotation  781S
781S
Sample solution:
50 mg/mL in water
Acceptance criteria:
 0.05
0.05 to +0.05
to +0.05
• Loss on Drying  731
731 :
Dry a sample in a vacuum over phosphorus pentoxide at 56
:
Dry a sample in a vacuum over phosphorus pentoxide at 56 for 4 h: it loses NMT 0.5% of its weight.
for 4 h: it loses NMT 0.5% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Huy T. Dinh, M.S.
Scientific Liaison 1-301-816-8594 |
(DS2010) Monographs - Dietary Supplements |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4208