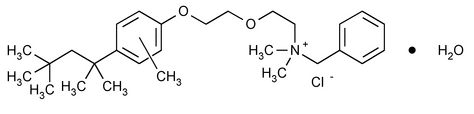
Methylbenzethonium Chloride
C28H44ClNO2·H2O 480.12
C28H44ClNO2 462.12
Benzenemethanaminium, N,N-dimethyl-N-[2-[2-[methyl-4-(1,1,3,3-tetramethylbutyl)phenoxy]ethoxy]ethyl]-, chloride, monohydrate;
Benzyldimethyl[2-[2-[[4-(1,1,3,3-tetramethylbutyl)tolyl]oxy]ethoxy]ethyl]ammonium chloride monohydrate

 [1320-44-1].
[1320-44-1].
Anhydrous

 [25155-18-4].
[25155-18-4].
(meth'' il ben'' ze thoe' nee um klor' ide).
C28H44ClNO2·H2O 480.12
C28H44ClNO2 462.12
Benzenemethanaminium, N,N-dimethyl-N-[2-[2-[methyl-4-(1,1,3,3-tetramethylbutyl)phenoxy]ethoxy]ethyl]-, chloride, monohydrate;
Benzyldimethyl[2-[2-[[4-(1,1,3,3-tetramethylbutyl)tolyl]oxy]ethoxy]ethyl]ammonium chloride monohydrate
Anhydrous
DEFINITION
Methylbenzethonium Chloride contains NLT 97.0% and NMT 103.0% of C28H44ClNO2, calculated on the dried basis.
IDENTIFICATION
• A. Procedure
Analysis:
To 1 mL of solution (1 in 100) add 2 mL of alcohol, 0.5 mL of 2 N nitric acid, and 1 mL of silver nitrate TS.
Acceptance criteria:
A white precipitate, which is insoluble in 2 N nitric acid and soluble in 6 N ammonium hydroxide, is formed.
ASSAY
• Procedure
Sample solution:
Transfer the equivalent to 500 mg of dried methylbenzethonium chloride, with the aid of 35 mL of water, to a glass-stoppered, 250-mL conical separator containing 25 mL of chloroform. Add 10.0 mL of freshly prepared potassium iodide solution (1 in 20), insert the stopper into the separator, shake, allow the layers to separate, and discard the chloroform layer. Wash the aqueous layer with three 10-mL portions of chloroform, and discard the washings. Transfer the aqueous layer to a glass-stoppered, 250-mL conical flask, and rinse the separator with three 5-mL portions of water, adding the washings to the flask. Add 40 mL of cold hydrochloric acid to the flask, and mix.
Analysis:
Titrate with 0.05 M potassium iodate VS until the solution becomes light brown in color. Add 5 mL of chloroform, insert the stopper into the flask, and shake vigorously. Continue the titration, dropwise, with shaking after each addition, until the chloroform layer becomes colorless and the aqueous layer is clear yellow. Perform a blank determination, using 20 mL of water as the test specimen (see Titrimetry  541
541 , Residual Titrations). Each mL of 0.05 M potassium iodate is equivalent to 46.21 mg of C28H44ClNO2.
, Residual Titrations). Each mL of 0.05 M potassium iodate is equivalent to 46.21 mg of C28H44ClNO2.
Acceptance criteria:
97.0%–103.0% on the dried basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.1%
:
NMT 0.1%
Organic Impurities
• Procedure: Limit of Ammonium Compounds
Analysis:
To 5 mL of a solution (1 in 50) add 3 mL of 1 N sodium hydroxide, and heat to boiling.
Acceptance criteria:
The odor of ammonia is not perceptible.
SPECIFIC TESTS
• Melting Range or Temperature  741
741 :
159
:
159 –163
–163 , the specimen having been previously dried
, the specimen having been previously dried
• Loss on Drying  731
731 :
Dry a sample at 105
:
Dry a sample at 105 for 4 h: it loses NMT 5.0% of its weight.
for 4 h: it loses NMT 5.0% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Leonel M. Santos, Ph.D.
Senior Scientific Liaison 1-301-816-8168 |
(SM12010) Monographs - Small Molecules 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3866
Pharmacopeial Forum: Volume No. 35(4) Page 853