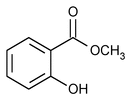
Methyl Salicylate
(meth' il sa lis' i late).
DEFINITION
Methyl Salicylate is produced synthetically or is obtained by maceration and subsequent distillation with steam from the leaves of Gaultheria procumbens Linné (Fam. Ericaceae) or from the bark of Betula lenta Linné (Fam. Betulaceae). It contains NLT 98.0% and NMT 100.5% of methyl salicylate (C8H8O3).
IDENTIFICATION
• A.
Sample:
1 drop of Methyl Salicylate
Analysis:
Shake the Sample with 5 mL of water, and add 1 drop of ferric chloride TS.
Acceptance criteria:
The resulting mixture has a deep violet color.
ASSAY
• Procedure
Sample:
2 g of Methyl Salicylate
Titrimetric system
(See Titrimetry  541
541 .)
.)
Mode:
Residual titration
Titrant:
1 N sodium hydroxide VS
Endpoint detection:
Colorimetric
Analysis:
Place the Sample in a flask, and add 40.0 mL of 1 N sodium hydroxide VS. Boil gently under a reflux condenser for 2 h. Cool, rinse the condenser and the sides of the flask with a few mL of water, and add phenolphthalein TS. Titrate the excess alkali with 1 N sulfuric acid VS. Perform a blank determination. Each mL of 1 N sodium hydroxide corresponds to 152.2 mg of methyl salicylate (C8H8O3).
Acceptance criteria:
98.0%–100.5%
IMPURITIES
• Heavy Metals, Method II  231
231 :
NMT 20 ppm
:
NMT 20 ppm
SPECIFIC TESTS
• Solubility in 70% Alcohol:
One volume of synthetic Methyl Salicylate dissolves in 7 volumes of 70% alcohol. One volume of natural Methyl Salicylate dissolves in 7 volumes of 70% alcohol, the solution having NMT a slight cloudiness.
• Specific Gravity  841
841 :
1.180–1.185 for the synthetic variety; 1.176–1.182 for the natural variety
:
1.180–1.185 for the synthetic variety; 1.176–1.182 for the natural variety
• Optical Rotation, Angular Rotation  781A
781A :
Synthetic Methyl Salicylate and that from Betula are optically inactive. Methyl Salicylate from Gaultheria is slightly levorotatory, the angular rotation not exceeding
:
Synthetic Methyl Salicylate and that from Betula are optically inactive. Methyl Salicylate from Gaultheria is slightly levorotatory, the angular rotation not exceeding  1.5
1.5 in a 100-mm tube.
in a 100-mm tube.
• Refractive Index  831
831 :
1.535–1.538 at 20
:
1.535–1.538 at 20
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers.
• Labeling:
Label it to indicate whether it was made synthetically or distilled from either of the plants of Gaultheria procumbens or Betula lenta.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S.
Scientific Liaison 1-301-816-8335 |
(EXC2010) Monographs - Excipients |
USP35–NF30 Page 1866