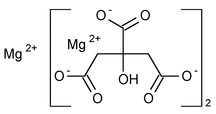
Magnesium Citrate Oral Solution
1,2,3-Propanetricarboxylic acid, hydroxy-, magnesium salt (2:3).
Magnesium citrate (3:2)
» Magnesium Citrate Oral Solution is a sterilized or pasteurized solution containing, in each 100 mL, not less than 7.59 g of anhydrous citric acid (C6H8O7) and an amount of magnesium citrate equivalent to not less than 1.55 g and not more than 1.9 g of magnesium oxide (MgO).
Magnesium Citrate Oral Solution may be prepared as follows:
| Magnesium Carbonate | 15 g |
| Anhydrous Citric Acid | 27.4 g |
| Syrup | 60 mL |
| Talc | 5 g |
| Lemon Oil | 0.1 mL |
| Potassium Bicarbonate | 2.5 g |
| Purified Water, a sufficient quantity, to make | 350 mL |
Dissolve the anhydrous Citric Acid in 150 mL of hot Purified Water in a suitable dish, slowly add the Magnesium Carbonate, previously mixed with 100 mL of Purified Water, and stir until it is dissolved. Then add the Syrup, heat the mixed liquids to the boiling point, immediately add the Lemon Oil, previously triturated with the Talc, and filter the mixture, while hot, into a strong bottle (previously rinsed with boiling Purified Water) of suitable capacity. Add boiled Purified Water to make the product measure 350 mL. Use Purified Cotton as a stopper for the bottle, allow to cool, add the Potassium Bicarbonate, and immediately insert the stopper in the bottle securely. Finally, shake the solution occasionally until the Potassium Bicarbonate is dissolved, cap the bottle, and sterilize or pasteurize the solution.
[note—An amount (30 g) of citric acid containing 1 molecule of water of hydration, equivalent to 27.4 g of anhydrous citric acid, may be used in the foregoing formula. In this process the 2.5 g of potassium bicarbonate may be replaced by 2.1 g of sodium bicarbonate, preferably in tablet form. The Oral Solution may be further carbonated by the use of carbon dioxide under pressure. ]
Packaging and storage—
Preserve at controlled room temperature or in a cool place, in bottles containing not less than 200 mL.
Identification—
A:
It responds to the tests for Magnesium  191
191 .
.
B:
To 5 mL of Oral Solution add 1 mL of potassium permanganate TS and 5 mL of mercuric sulfate TS, and heat the solution: a white precipitate is formed.
Chloride  221
221 —
A 2.0-mL portion shows no more chloride than corresponds to 0.30 mL of 0.020 N hydrochloric acid (0.01%).
—
A 2.0-mL portion shows no more chloride than corresponds to 0.30 mL of 0.020 N hydrochloric acid (0.01%).
Sulfate  221
221 —
A 2.0-mL portion shows no more sulfate than corresponds to 0.30 mL of 0.020 N sulfuric acid (0.015%).
—
A 2.0-mL portion shows no more sulfate than corresponds to 0.30 mL of 0.020 N sulfuric acid (0.015%).
Tartaric acid—
To 10 mL in a test tube add 1 mL of glacial acetic acid and 3 mL of a solution of potassium acetate (1 in 2), shake the mixture vigorously, then gently rub the inner wall of the test tube with a glass rod for a few minutes, and allow to stand for 1 hour: no white, crystalline precipitate soluble in 6 N ammonium hydroxide is formed.
Assay for anhydrous citric acid—
Mobile Phase, Standard Preparation 1, and Chromatographic System—
Proceed as directed under Assay for Citric Acid/Citrate and Phosphate  345
345 .
.
Assay preparation—
Measure accurately 10 mL of Oral Solution that previously has been freed from excessive carbon dioxide by repeated pouring, into a suitable volumetric flask, and proceed as directed for Assay Preparation for Citric Acid/Citrate Assay under Assay for Citric Acid/Citrate and Phosphate  345
345 .
.
Procedure—
Proceed as directed for Procedure under  345
345 , and calculate the quantity, in mg, of anhydrous citric acid (C6H8O7) in the volume of Oral Solution taken by the formula:
, and calculate the quantity, in mg, of anhydrous citric acid (C6H8O7) in the volume of Oral Solution taken by the formula:
0.001(192.12 / 189.10)CSD(rU / rS)
in which 192.12 is the molecular weight of anhydrous citric acid; 189.10 is the molecular weight of citrate (C6H5O7); CS is the concentration, in µg per mL, of citrate in Standard Preparation 1; D is the dilution factor; and rU and rS are the citrate peak areas obtained from the Assay preparation and Standard Preparation 1, respectively.
Assay for magnesium oxide—
Transfer to a 100-mL volumetric flask 50.0 mL of Oral Solution that has been previously freed from excessive carbon dioxide by repeated pouring. Dilute with water to volume, and mix. Transfer 5.0 mL of this solution to a beaker containing 150 mL of water heated to 70 to 80
to 80 , and add 1 mL of ammonium chloride TS and then 3 mL of ammonium hydroxide. Mix, and add slowly, with stirring, 8 mL of 8-hydroxyquinoline TS. After standing for 30 minutes, filter through a sintered-glass crucible, previously dried and weighed, and wash the precipitate with ten 10-mL portions of water. Dry the crucible and contents at 105
, and add 1 mL of ammonium chloride TS and then 3 mL of ammonium hydroxide. Mix, and add slowly, with stirring, 8 mL of 8-hydroxyquinoline TS. After standing for 30 minutes, filter through a sintered-glass crucible, previously dried and weighed, and wash the precipitate with ten 10-mL portions of water. Dry the crucible and contents at 105 for 3 hours, cool, and weigh. Determine the equivalent of MgO in 100 mL of the Oral Solution by multiplying the weight of C18H12MgN2O2·2H2O so obtained by 4.624.
for 3 hours, cool, and weigh. Determine the equivalent of MgO in 100 mL of the Oral Solution by multiplying the weight of C18H12MgN2O2·2H2O so obtained by 4.624.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Jeanne H. Sun
Assistant Scientific Liaison 1-301-816-3361 |
(CMP2010) Compounding |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3746
Pharmacopeial Forum: Volume No. 31(2) Page 420