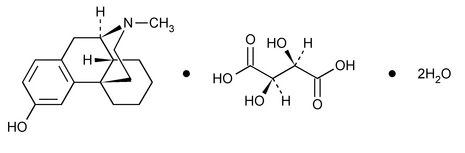
Levorphanol Tartrate
(lee vor' fa nol tar' trate).
Morphinan-3-ol, 17-methyl-, [R-(R*,R*)]-2,3-dihydroxybutanedioate (1:1) (salt), dihydrate.
17-Methylmorphinan-3-ol, tartrate (1:1) (salt) dihydrate
Anhydrous 407.47
» Levorphanol Tartrate contains not less than 99.0 percent and not more than 101.0 percent of C17H23NO·C4H6O6, calculated on the anhydrous basis.
Packaging and storage—
Preserve in well-closed containers. Store at 25 , excursions permitted between 15
, excursions permitted between 15 and 30
and 30 .
.
Identification—
A:
Infrared Absorption  197K
197K —Obtain the test specimen as follows. Dissolve 50 mg in 25 mL of water in a 125-mL separator. Add 2 mL of 6 N ammonium hydroxide, extract with 25 mL of chloroform, and filter the chloroform extract through a layer of 4 g of granular anhydrous sodium sulfate supported on glass wool into a 125-mL conical flask. Evaporate the chloroform extract on a steam bath with the aid of a stream of nitrogen to dryness. Dissolve the residue in 1 mL of acetone, and evaporate to dryness. Dry in vacuum at 90
—Obtain the test specimen as follows. Dissolve 50 mg in 25 mL of water in a 125-mL separator. Add 2 mL of 6 N ammonium hydroxide, extract with 25 mL of chloroform, and filter the chloroform extract through a layer of 4 g of granular anhydrous sodium sulfate supported on glass wool into a 125-mL conical flask. Evaporate the chloroform extract on a steam bath with the aid of a stream of nitrogen to dryness. Dissolve the residue in 1 mL of acetone, and evaporate to dryness. Dry in vacuum at 90 for 1 hour. Proceed as directed with the dried levorphanol so obtained and a similar preparation of USP Levorphanol Tartrate RS.
for 1 hour. Proceed as directed with the dried levorphanol so obtained and a similar preparation of USP Levorphanol Tartrate RS.
B: Ultraviolet Absorption  197U
197U —
—
Solution:
130 µg per mL.
Medium:
0.1 N hydrochloric acid.
Absorptivities at 279 nm, calculated on the anhydrous basis, do not differ by more than 3.0%.
Specific rotation  781S
781S :
between
:
between  14.7
14.7 and
and  16.3
16.3 .
.
Test solution:
30 mg per mL, in water. Heat on a water bath or sonicate to dissolve 750 mg in 20 mL of water in a 25-mL volumetric flask, dilute with water to volume, and mix.
Water, Method I  921
921 :
between 7.0% and 9.0%.
:
between 7.0% and 9.0%.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Ordinary impurities  466
466 —
—
Test solution:
water.
Standard solution:
water.
Eluant:
a mixture of hexanes, dehydrated alcohol, and ammonium hydroxide (80:25:1).
Visualization:
17; then view immediately under short-wavelength UV light.
Assay—
Dissolve about 900 mg of sample, accurately weighed, in 85 mL of glacial acetic acid, warming slightly if necessary. Titrate with 0.1 N perchloric acid VS and determine the endpoint potentiometrically. Perform a blank determination and make any nececssary corrections. Each mL of 0.1 N perchloric acid consumed by the sample is equivalent to 40.75 mg of C17H23NO·C4H6O6.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Clydewyn M. Anthony, Ph.D.
Senior Scientific Liaison 1-301-816-8139 |
(SM22010) Monographs - Small Molecules 2 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3675
Pharmacopeial Forum: Volume No. 34(2) Page 280