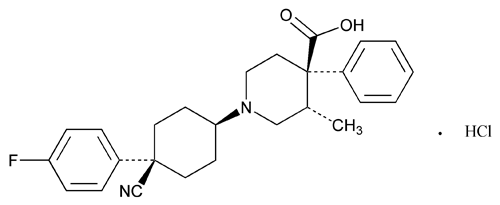
Levocabastine Hydrochloride
C26H29FN2O2·HCl 456.98
4-Piperidinecarboxylic acid, 1-[4-cyano-4-(4-fluorophenyl)cyclohexyl]-3-methyl-4-phenyl-, monohydrochloride, (–)-[1(cis),3 ,4
,4 ]-;
]-;
(–)-trans-1-[cis-4-Cyano-4-(p-fluorophenyl)cyclohexyl]-3-methyl-4-phenylisonipecotic acid monohydrochloride

 [79547-78-7].
[79547-78-7].
(lee'' voe ka bas' teen hye'' droe klor' ide).
C26H29FN2O2·HCl 456.98
4-Piperidinecarboxylic acid, 1-[4-cyano-4-(4-fluorophenyl)cyclohexyl]-3-methyl-4-phenyl-, monohydrochloride, (–)-[1(cis),3
(–)-trans-1-[cis-4-Cyano-4-(p-fluorophenyl)cyclohexyl]-3-methyl-4-phenylisonipecotic acid monohydrochloride
DEFINITION
Levocabastine Hydrochloride contains NLT 98.5% and NMT 101.5% of C26H29FN2O2·HCl, calculated on the dried basis.
IDENTIFICATION
• B. Identification Tests—General, Chloride  191
191 :
Meets the requirements
:
Meets the requirements
• C. Optical Rotation, Specific Rotation  781S
781S :
Meets the requirements
:
Meets the requirements
ASSAY
• Procedure
Sample solution:
Dissolve 175 mg of Levocabastine Hydrochloride in 50 mL of alcohol, and add 5.0 mL of 0.01 N hydrochloric acid.
Titrimetric system
(See Titrimetry  541
541 .)
.)
Mode:
Direct titration
Titrant:
0.1 N sodium hydroxide VS
Endpoint detection:
Potentiometric
Analysis
Sample:
Sample solution
The volume of titrant required to titrate Levocabastine Hydrochloride is the difference between the first and third endpoints. Perform a blank determination and make any necessary correction. Each mL of 0.1 N sodium hydroxide VS is equivalent to 22.85 mg of C26H29FN2O2·HCl.
Acceptance criteria:
98.5%–101.5% on the dried basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.1%, based on a sample weight of about 1.000 g
:
NMT 0.1%, based on a sample weight of about 1.000 g
Organic Impurities
• Procedure
[Note—Prepare solutions immediately before use. ]
Diluent:
2 mg/mL of sodium hydroxide in water
Solution A:
Dissolve 1.39 g of boric acid in water, and adjust with 1 N sodium hydroxide to a pH of 9.0. Dilute with water to 100 mL.
Run buffer:
Dissolve 1.08 g of sodium dodecyl sulfate and 650 mg of hydroxypropyl- -cyclodextrin in 5 mL of isopropyl alcohol, then dilute with Solution A to 50 mL.
-cyclodextrin in 5 mL of isopropyl alcohol, then dilute with Solution A to 50 mL.
System suitability solution:
12.5 µg/mL of USP Levocabastine Hydrochloride RS and 12.5 µg/mL of USP Levocabastine Related Compound A RS in Diluent
Standard solution:
Dilute 5.0 mL of the Sample solution with Diluent to 100 mL. Dilute 1.0 mL of this solution with Diluent to 10 mL to obtain a solution containing 12.5 µg/mL of Levocabastine Hydrochloride.
Sample solution:
2.5 mg/mL of Levocabastine Hydrochloride in Diluent
Capillary electrophoresis system
Detector:
UV 214 nm
Column:
75-µm × 50-cm uncoated fused-silica capillary column
Column temperature:
50
Current:
See the gradient table below.
| Time (min) |
Current (µA) |
|---|---|
| 0 | 0 |
| 0.17 | 75 |
| 15 | 130 |
| 40 | 130 |
| 60 | 200 |
[Note—Before performing the System suitability, equilibrate the capillary column with Diluent for 2 min, then equilibrate with Run buffer for at least 5 min. ]
System suitability
Sample:
System suitability solution
[Note—The relative migration times for levocabastine and levocabastine related compound A are approximately 1.0 and 1.07, respectively. ]
Suitability requirements
Resolution:
NLT 4 between levocabastine and levocabastine related compound A
[Note—If necessary, adjust the current gradient to achieve the required resolution. ]
Analysis
Samples:
Diluent (blank), Standard solution, and Sample solution
Separately inject equal volumes (pressure of 3450 Pa for 5 s) of the Samples, and record the peak responses.
[Note—Disregard any peak originating from the Diluent. Disregard any peak with an area of less than 0.1 times the major peak area of the Standard solution (0.05%). ]
Acceptance criteria:
The area for any peak in the Sample solution, other than the major peak, is not greater than the major peak area of the Standard solution (0.5%); and the sum of all peak areas in the Sample solution, except for the major peak, is not greater than twice the major peak area of the Standard solution (1.0%).
SPECIFIC TESTS
• Loss on Drying  731
731 :
Dry about 1.000 g of the sample at 105
:
Dry about 1.000 g of the sample at 105 to constant weight: it loses NMT 0.5% of its weight.
to constant weight: it loses NMT 0.5% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers. Protect from light.
• USP Reference Standards  11
11
USP Levocabastine Related Compound A RS
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Mary S. Waddell
Scientific Liaison 1-301-816-8124 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3664
Pharmacopeial Forum: Volume No. 36(1) Page 116