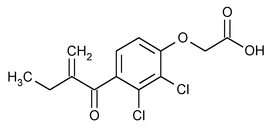
Ethacrynic Acid
(eth a krin' ik as' id).
Acetic acid, [2,3-dichloro-4-(2-methylene-1-oxobutyl)phenoxy]-.
[2,3-Dichloro-4-(2-methylenebutyryl)phenoxy]acetic acid
» Ethacrynic Acid contains not less than 97.0 percent and not more than 102.0 percent of C13H12Cl2O4, calculated on the dried basis.
[Caution—Use care in handling Ethacrynic Acid, since it irritates the skin, eyes, and mucous membranes.
]
Packaging and storage—
Preserve in well-closed containers. Store at 25 , excursions permitted between 15
, excursions permitted between 15 and 30
and 30 .
.
Identification—
B: Ultraviolet Absorption  197U
197U —
—
Solution:
50 µg per mL.
Medium:
methanol.
Absorptivities at 271 nm, calculated on the dried basis, do not differ by more than 3.0%.
C:
Add 2 mL of 1 N sodium hydroxide to about 25 mg of it, and heat for several minutes in a boiling water bath. Cool the solution, acidify with 0.25 mL of 18 N sulfuric acid, add 0.5 mL of chromotropic acid sodium salt solution (1 in 10), then add, cautiously, 2 mL of sulfuric acid TS: a deep violet color is produced.
Loss on drying  731
731 —
Dry it at a pressure not exceeding 5 mm of mercury at 60
—
Dry it at a pressure not exceeding 5 mm of mercury at 60 for 2 hours: it loses not more than 0.25% of its weight.
for 2 hours: it loses not more than 0.25% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Toluene extractives—
Accurately weigh about 1 g into a glass-stoppered, 100-mL cylinder. Add 50 mL of sodium sulfite solution (2 in 25), and agitate until the solid dissolves. Allow to stand for 20 minutes, add 5 mL of hydrochloric acid, and mix. Divide the solution between two centrifuge tubes, each of which contains 15 mL of toluene. Close each tube tightly, using a polyethylene stopper, and shake vigorously during 2 minutes, occasionally relieving the pressure from the sulfur dioxide by loosening the stoppers. Centrifuge the tubes, withdraw most of the upper layer by means of a syringe, avoiding withdrawal of any of the lower, aqueous phase, and transfer the toluene extracts to a tared evaporating dish. Repeat the extraction twice with additional 15-mL portions of toluene, and evaporate the combined extracts on a steam bath to dryness. Dry the residue at a pressure not exceeding 5 mm of mercury at 60 for 2 hours. Cool, and weigh: not more than 2.0% of extractives is found.
for 2 hours. Cool, and weigh: not more than 2.0% of extractives is found.
Equivalent weight—
Dissolve about 400 mg, accurately weighed, in 100 mL of methanol, add 5 mL of water, and titrate with 0.1 N sodium hydroxide VS, determining the endpoint potentiometrically, using a calomel–glass electrode system. Perform a blank determination, and make any necessary correction. Calculate the equivalent weight on the dried basis: it is between 294 and 309.
Heavy metals, Method II  231
231 :
0.001%.
:
0.001%.
Assay—
Dissolve about 100 mg of Ethacrynic Acid, accurately weighed, in 20 mL of glacial acetic acid in an iodine flask. Pipet 20 mL of 0.1 N bromine VS into the flask, add 3 mL of hydrochloric acid, immediately insert the stopper, and seal the flask with a few mL of water in the stopper well. Swirl the flask, and allow to stand in the dark for 1 hour. Add 50 mL of water and 15 mL of potassium iodide TS, and immediately titrate with 0.1 N sodium thiosulfate VS, adding 2.0 mL of starch TS as the endpoint is approached. Perform a blank determination (see Residual Titrations under Titrimetry  541
541 ). Each mL of 0.1 N bromine is equivalent to 15.16 mg of C13H12Cl2O4.
). Each mL of 0.1 N bromine is equivalent to 15.16 mg of C13H12Cl2O4.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Sujatha Ramakrishna, Ph.D.
Senior Scientific Liaison 1-301-816-8349 |
(SM22010) Monographs - Small Molecules 2 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3121
Pharmacopeial Forum: Volume No. 29(5) Page 1479