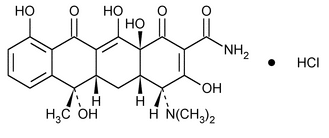
Epitetracycline Hydrochloride
(ep'' i tet'' ra sye' kleen hye'' droe klor' ide).
2-Naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-, monohydrochloride, [4R-(4
(4R,4aS,5aS,6S,12aS) 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide monohydrochloride
» Epitetracycline Hydrochloride contains not less than 70.0 percent of C22H24N2O8·HCl.
Packaging and storage—
Preserve in tight, light-resistant containers.
pH  791
791 :
between 2.3 and 4.0, in a solution containing 10 mg per mL.
:
between 2.3 and 4.0, in a solution containing 10 mg per mL.
Loss on drying  731
731 —
Dry about 100 mg in a capillary-stoppered bottle in vacuum at a pressure not exceeding 5 mm of mercury at 60
—
Dry about 100 mg in a capillary-stoppered bottle in vacuum at a pressure not exceeding 5 mm of mercury at 60 for 3 hours: it loses not more than 6.0% of its weight.
for 3 hours: it loses not more than 6.0% of its weight.
4-Epianhydrotetracycline  226
226 —
Dissolve about 250 mg, accurately weighed, in 10 mL of 0.1 N hydrochloric acid, and adjust with 6 N ammonium hydroxide to a pH of 7.8. Transfer this solution with the aid of EDTA buffer to a 50-mL volumetric flask, dilute with EDTA buffer to volume, and mix. Use this solution, without delay, as the Test solution: not more than 2.0% is found.
—
Dissolve about 250 mg, accurately weighed, in 10 mL of 0.1 N hydrochloric acid, and adjust with 6 N ammonium hydroxide to a pH of 7.8. Transfer this solution with the aid of EDTA buffer to a 50-mL volumetric flask, dilute with EDTA buffer to volume, and mix. Use this solution, without delay, as the Test solution: not more than 2.0% is found.
Assay—
Edetate disodium solution
, Stationary phase, Alkaline methanol solution, Column support, Chromatographic column, and Standard preparation—Prepare as directed in the section Epitetracycline hydrochloride content and Assay for tetracycline hydrochloride under Tetracycline Hydrochloride for Topical Solution.
Assay preparation—
Transfer about 22 mg of Epitetracycline Hydrochloride, accurately weighed, to a 25-mL volumetric flask, add 1 mL of methanol, and swirl to dissolve. Dilute with Stationary phase to volume, and mix. Transfer 2.0 mL of this solution to a 10-mL volumetric flask, dilute with Stationary phase to volume, and mix. Pipet 2.0 mL of this solution into the Chromatographic column, and allow it to penetrate the Column support. Add 20 mL of benzene to the solvent reservoir, and collect the eluate at the rate of about 1 mL per minute. When the benzene level reaches the top of the Column support, add 60 mL of chloroform to the solvent reservoir, and continue collecting eluate. When the chloroform level reaches the top of the Column support, discard the collected eluate, add 50 mL of a mixture of butyl alcohol and methanol (1:1), and collect 8 mL of eluate in a 10-mL graduated cylinder. Replace the 10-mL graduated cylinder with a low-actinic 50-mL volumetric flask, and continue collecting eluate until the column runs dry. The eluate in the 50-mL volumetric flask is the Assay preparation.
Procedure—
Add 2.0 mL of Alkaline methanol solution to the Standard preparation and to the Assay preparation, dilute each with chloroform to volume, and mix. Concomitantly, within 10 minutes of preparation, determine the absorbances of these solutions at the wavelength of maximum absorbance at about 366 nm, with a suitable spectrophotometer, using chloroform as the blank. Calculate the quantity, in mg, of C22H24N2O8·HCl in the Epitetracycline Hydrochloride taken by the formula:
0.001(WP)(AU / AS)
in which W is the weight, in mg, of USP Tetracycline Hydrochloride RS taken, P is the potency, in µg per mg, of the USP Tetracycline Hydrochloride RS, and AU and AS are the absorbances of the solutions from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ahalya Wise, M.S.
Senior Scientific Liaison 1-301-816-8161 |
(SM12010) Monographs - Small Molecules 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3059