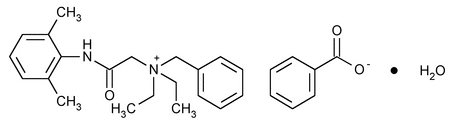Denatonium Benzoate
(den'' a toe' nee um ben' zoe ate).
Benzenemethanaminium, N-[2-[(2,6-dimethylphenyl)amino]-2-oxoethyl]-N,N-diethyl-, benzoate, monohydrate.
Benzyldiethyl[(2,6-xylylcarbamoyl)methyl]ammonium benzoate monohydrate
Anhydrous 446.59
» Denatonium Benzoate, dried at 105 for 2 hours, contains one molecule of water of hydration or is anhydrous. When dried at 105
for 2 hours, contains one molecule of water of hydration or is anhydrous. When dried at 105 for 2 hours, it contains not less than 99.5 percent and not more than 101.0 percent of C28H34N2O3.
for 2 hours, it contains not less than 99.5 percent and not more than 101.0 percent of C28H34N2O3.
Packaging and storage—
Preserve in tight containers.
Labeling—
Label it to indicate whether it is hydrous or anhydrous.
Identification—
Solution:
100 µg per mL.
Medium:
water.
Absorptivities at 263 nm, calculated on the dried basis, do not differ by more than 3.0%.
C:
Dissolve about 150 mg in 10 mL of water, and add 15 mL of trinitrophenol TS: a yellow precipitate is formed.
D:
Dissolve about 100 mg in 10 mL of water, and add 20 mL of 2 N sulfuric acid and 15 mL of ammonium reineckate TS. Mix, filter through a sintered-glass crucible using gentle suction, and wash thoroughly with water. Remove as much water as possible with suction, and then dry in an oven at 105 for 1 hour: the denatonium reineckate so obtained melts at about 170
for 1 hour: the denatonium reineckate so obtained melts at about 170 (see Melting Range or Temperature
(see Melting Range or Temperature  741
741 ).
).
Melting range  741
741 :
between 163
:
between 163 and 170
and 170 , on a dried specimen.
, on a dried specimen.
pH  791
791 :
between 6.5 and 7.5, in a solution (3 in 100).
:
between 6.5 and 7.5, in a solution (3 in 100).
Loss on drying  731
731 —
Dry it at 105
—
Dry it at 105 for 2 hours: the monohydrate loses between 3.5% and 4.5% of its weight, and the anhydrous form loses not more than 1.0% of its weight.
for 2 hours: the monohydrate loses between 3.5% and 4.5% of its weight, and the anhydrous form loses not more than 1.0% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Chloride  221
221 —
Dissolve 350 mg in 9 mL of water, add 1 mL of nitric acid, and filter. A 1.0-mL portion of the filtrate shows no more chloride than corresponds to 0.10 mL of 0.020 N hydrochloric acid (0.2%).
—
Dissolve 350 mg in 9 mL of water, add 1 mL of nitric acid, and filter. A 1.0-mL portion of the filtrate shows no more chloride than corresponds to 0.10 mL of 0.020 N hydrochloric acid (0.2%).
Assay—
Dissolve about 900 mg of Denatonium Benzoate, previously dried and accurately weighed, in 50 mL of glacial acetic acid, add 1 drop of crystal violet TS, and titrate with 0.1 N perchloric acid VS to a green endpoint. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 44.66 mg of C28H34N2O3.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S.
Scientific Liaison 1-301-816-8335 |
(EXC2010) Monographs - Excipients |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1778