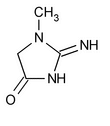Creatinine
(kree at' i neen).
DEFINITION
Creatinine contains NLT 98.5% and NMT 102.0% of C4H7N3O, as Creatinine, calculated on the dried basis.
IDENTIFICATION
ASSAY
• Procedure
Sample:
40 mg of Creatinine
Titrimetric system
(See Titrimetry  541
541 .)
.)
Mode:
Direct titration
Titrant:
0.1 N perchloric acid VS
Endpoint detection:
Potentiometric
Indicator electrode:
Glass
Reference electrode:
Silver–silver chloride
Reference electrode solution:
Saturated lithium perchlorate and silver chloride in glacial acetic acid
Analysis:
Dissolve the Sample in 10 mL of glacial acetic acid. Titrate the Sample with 0.1 N perchloric acid VS. Perform a blank determination, and make any necessary correction.
Calculate the percentage of creatinine (C4H7N3O) in the Sample taken:
Result = [(V  B) × N × F × 100]/W
B) × N × F × 100]/W
| V | = | = volume of the Titrant consumed by the Sample (mL) |
| B | = | = volume of the Titrant consumed by the Blank (mL) |
| N | = | = actual normality of the Titrant (mEq/mL) |
| F | = | = equivalency factor, 113.12 mg/mEq |
| W | = | = weight of the Sample (mg) |
Acceptance criteria:
98.5%–102.0% on the dried basis
IMPURITIES
• Residue on Ignition  281
281 :
NMT 0.2%
:
NMT 0.2%
• Heavy Metals, Method I  231
231 :
10 ppm
:
10 ppm
SPECIFIC TESTS
• Loss on Drying  731
731 :
Dry a sample at 105
:
Dry a sample at 105 for 3 h: it loses NMT 3.0% of its weight.
for 3 h: it loses NMT 3.0% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S.
Scientific Liaison 1-301-816-8335 |
(EXC2010) Monographs - Excipients |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1773