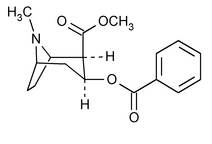
Cocaine
(koe kane').
8-Azabicyclo[3.2.1]octane-2-carboxylic acid, 3-(benzoyloxy)-8-methyl-, methyl ester, [1R-(exo,exo)]-.
Methyl 3
» Cocaine, dried over phosphorus pentoxide for 3 hours, contains not less than 99.0 percent and not more than 101.0 percent of C17H21NO4.
Packaging and storage—
Preserve in well-closed, light-resistant containers.
Identification—
A:
Ultraviolet Absorption  197U
197U —
—
Solution:
15 µg per mL.
Medium:
dilute hydrochloric acid (1 in 120).
Absorptivities at 233 nm, calculated on the dried basis, do not differ by more than 3.0%.
B:
It meets the requirements under Identification—Organic Nitrogenous Bases  181
181 , USP Cocaine Hydrochloride RS being used, and sodium carbonate TS being used in place of sodium hydroxide TS.
, USP Cocaine Hydrochloride RS being used, and sodium carbonate TS being used in place of sodium hydroxide TS.
C:
Dissolve about 100 mg in a mixture of 0.4 mL of dilute hydrochloric acid (1 in 12) and water to make 5 mL, and add 5 drops of chromium trioxide solution (1 in 20): a yellow precipitate is formed, and it quickly redissolves when the mixture is shaken. Add 1 mL of hydrochloric acid: a permanent, orange-colored, crystalline precipitate is formed.
D:
Dissolve about 10 mg in 1 mL of dilute hydrochloric acid (1 in 600), and evaporate on a steam bath just to dryness. Dissolve the residue in 2 drops of water, and add 1 mL of potassium permanganate solution (1 in 300): a violet, crystalline precipitate is formed, and it appears brownish violet when collected on a filter, and shows characteristic violet-red crystalline aggregates under the low power of a microscope, similar to those obtained from USP Cocaine Hydrochloride RS.
Melting range, Class I  741
741 :
between 96
:
between 96 and 98
and 98 .
.
Loss on drying  731
731 —
Dry it over phosphorus pentoxide for 3 hours: it loses not more than 1.0% of its weight.
—
Dry it over phosphorus pentoxide for 3 hours: it loses not more than 1.0% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Readily carbonizable substances  271
271 —
Dissolve about 500 mg in 5 mL of sulfuric acid: the solution has no more color than Matching Fluid A.
—
Dissolve about 500 mg in 5 mL of sulfuric acid: the solution has no more color than Matching Fluid A.
Limit of cinnamyl-cocaine and other reducing substances—
Dissolve about 300 mg of finely powdered Cocaine in 1 mL of dilute hydrochloric acid (1 in 12) with the aid of heat, if necessary, and dilute with water to 15 mL. Mix 5 mL of this solution with 0.3 mL of dilute sulfuric acid (1 in 35) and 0.1 mL of potassium permanganate solution (1 in 300): the violet color does not disappear entirely within 30 minutes.
Limit of isoatropyl-cocaine—
Dilute in a beaker 5 mL of the solution of Cocaine prepared in the test for Cinnamyl-cocaine and other reducing substances with 80 mL of water, add 0.2 mL of 6 N ammonium hydroxide, and stir the solution vigorously for 5 minutes, occasionally rubbing the inner wall of the beaker with a stirring rod: a crystalline precipitate of cocaine is formed, and the supernatant is clear.
Assay—
Dissolve about 600 mg of Cocaine, previously dried and accurately weighed, in 50 mL of glacial acetic acid, add 1 drop of crystal violet TS, and titrate with 0.1 N perchloric acid VS to a green endpoint. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 30.34 mg of C17H21NO4.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Maged H. Sharaf, Ph.D.
Principal Scientific Liaison 1-301-816-8318 |
(DS2010) Monographs - Dietary Supplements |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2753
Pharmacopeial Forum: Volume No. 34(5) Page 1151